Prevalence of Schistosomes and Soil-Transmitted Helminths among Schoolchildren in Lake Victoria Basin, Tanzania
Article information
Abstract
The objectives of this study was to conduct a survey on schistosomiasis and soil-transmitted helminth (STH) infections in order to come up with feasible control strategies in Lake Victoria basin, Tanzania. Depending on the size of the school, 150-200 schoolchildren were recruited for the study. Duplicate Kato-Katz stool smears were prepared from each child and microscopically examined for Schistosoma mansoni and STHs. Urine specimens were examined for Schistosoma haematobium eggs using the filtration technique. After the survey, mass drug administration was done using praziquantel and albendazole for schistosomiasis and STHs infections, respectively. A total of 5,952 schoolchildren from 36 schools were recruited for the study and had their stool and urine specimens examined. Out of 5,952 schoolchildren, 898 (15.1%) were positive for S. mansoni, 754 (12.6%) for hookworms, 188 (3.2%) for Ascaris lumblicoides, and 5 (0.008%) for Trichuris trichiura. Out of 5,826 schoolchildren who provided urine samples, 519 (8.9%) were positive for S. haematobium eggs. The results revealed that intestinal schistosomiasis, urogenital schistosomiasis, and STH infections are highly prevalent throughought the lake basin. The high prevalence of intestinal and urogenital schistosomisiasis in the study area was a function of the distance from Lake Victoria, the former being more prevalent at localities close to the lake, whilst the latter is more so away from it. Control of schistosomiasis and STHs in the study area requires an integrated strategy that involves provision of health education to communities, regular treatments, and provision of adequate safe water supply and sanitation facilities.
INTRODUCTION
World Health Organization (WHO) estimated that over 237 million people required treatment for schistosomiasis in 2010 [1] with estimates of up to an additional 779 million at risk globally [2]. Schistosomiasis is endemic in 76 countries worldwide and besides malaria is the second important parasitic disease for public health [3,4]. Of the 662 million people infected worldwide, 85% are from Africa [5]. In Tanzania, schistosomiasis is highly endemic, and its prevalence varies from one region to another, with a prevalence of up to 80% in highly endemic areas [6]. Intestinal schistosomiasis is caused by Schistosoma mansoni, and infections are acquired by contact with freshwater containing parasite larvae. The disease is hyper-endemic in the Great Lake regions of East Africa, owing to the favorable habitat for snails of the Biomphalaria genus, which are the intermediate host [7]. Recent studies around Lake Victoria indicated that the prevalence in schoolchildren vary widely [8-12]. In endemic areas, initial infection is acquired at a young age. Verani et al. [13] found that 14% of 1-year-old children along the Kenyan shores of Lake Victoria were S. mansoni positive, whereas Odogwu et al. [14] found a prevalence of 47.4% in children <3 years of age around Lake Victoria in Uganda. The prevalence in school-aged children in these areas could be as high as 86-90% [15].
In children, schistosomiasis normally presents with generalized, non-specific signs and symptoms, making it difficult to identify disease-specific morbidity indicators and challenging to develop tools for assessing those indicators. Over time, morbidity may progress from subtle manifestations such as anemia, to severe, debilitating, and irreversible conditions such as growth stunting, impaired cognitive development, increased susceptibility to co-infection, decreased quality of life, exercise intolerance, infertility, portal hypertension, and liver failure [16-22].
WHO estimated that more than a billion people are chronically infected with soil-transmitted helminths (STHs) [23]. Ascaris lumbricoides, hookworms (Ancylostoma duodenale and Necator americanus), and Trichuris trichiura are the most common STH species with global prevalence of about 1,000, 900-1,300, and 500 million people, respectively [24-26]. As such, STH infections are still considered to be the most prevalent infections of mankind. Nowadays, STH has been classified among the most prevalent neglected tropical diseases (NTDs) as they persist exclusively in the poorest populations [27].
Often, schistosome infections co-occur with STHs. Findings from a study done among schoolchildren in a Lake Victoria shore line ward in Tanzania showed that the prevalence of hookworms was the second to intestinal schistosomiasis [6,28]. In Kenya, the prevalence of STHs is prominently attributed to A. lumbricoides, hookworms, and T. trichiura [29,30]. Hookworm infections observed in the lake basin in both Tanzania and Kenya were similarly high, ranging from 37.0-42.5% [10,31]. Other studies in Magu District, Tanzania, reported a prevalence of ascariasis of <1% [10], and Handzel et al. [31] reported in Kenya's Nyanza Province the prevalence of 22.9% and 17.9% for A. lumbricoides and T. trichuris, respectively.
Possible factors associated with STH infections among the studied children included age (school-age), absence of toilet and piped water supply in the household, large family size (≥7 members), and not washing hands before eating and after defecation [32].
Although helminths can infect all members of a population, there are specific groups who are more vulnerable and at a greater risk of infection [33,34]. For schistosomes and STHs, the most vulnerable groups are preschool children, school-aged children, and women of child-bearing age, including adolescent girls although much of the morbidity associated with infection can be reversed with the use of effective anthelmintic drug treatments [35,36].
Preschool children and school-aged children including adolescents tend to harbor the greatest numbers of intestinal worms and schistosomes and as a result experience growth stunting and diminished physical fitness as well as impaired memory and cognition [37]. These adverse health consequences combine to impair childhood educational performance, reduce school attendance [37], with hookworms being well-known causes of anemia because of intestinal blood loss [38].
Chemotherapy with praziquantel (PZQ) is currently the mainstay of control, which is available at a low cost [39,40]. Moreover, due to their geographical overlap between schistosomes and STH infections, they could be simultaneously treated with 2 drugs, albendazole and praziquantel [41]. As such, the successful STH control programmes have enormous benefits of improved nutritional and health status of the children, higher educational attainment, labor force participation, productivity, and income among the most vulnerable populations [42-44]. An added externality is the impact that NTDs have on HIV/AIDS, tuberculosis, and malaria. Several recent papers highlight the immunosuppressive features of helminths (especially the STHs, schistosomes, and filariae) and their possible impact on promoting susceptibility to the big 3 diseases [45,46]. New data suggest that control of NTDs could become a powerful tool for combating HIV/AIDS, tuberculosis, and malaria [47]. This paper presents data using a rapid assessment methodology to examine the prevalence of schistosomiasis and STH infections in schools around the Tanzania perimeter of Lake Victoria.
MATERIALS AND METHODS
Study area and population
The study area is located on the northwest of Tanzania around Lake Victoria (Fig. 1). It stretches from the southwest of the lake through south to the eastern side. The area is comprised of Kagera, Mwanza, Mara on the lake shore, and Shinyanga about 60 km away from the lake. The study area is bordered by Uganda and Rwanda in the north and west, respectively. It is also bordered by Tabora and Manyara regions in the south and east, respectively. The main ethinic groups are; Wahaya and Wasubi in Kagera region, Wasukuma, Wazinza, and Wasumbwa in Mwanza region, Wajita, Wakurya, and Waluo in Mara region, and again Wasukuma and Wasumbwa in Shinyanga region. The major occupations of these people are peasant farming, pastoralism, and fishing. Much of the activities have a bearing to schistosomiasis and STH infections as they performed without protective gear in the area where the level of sanitation and hygiene is as low as in any poor resource settings.
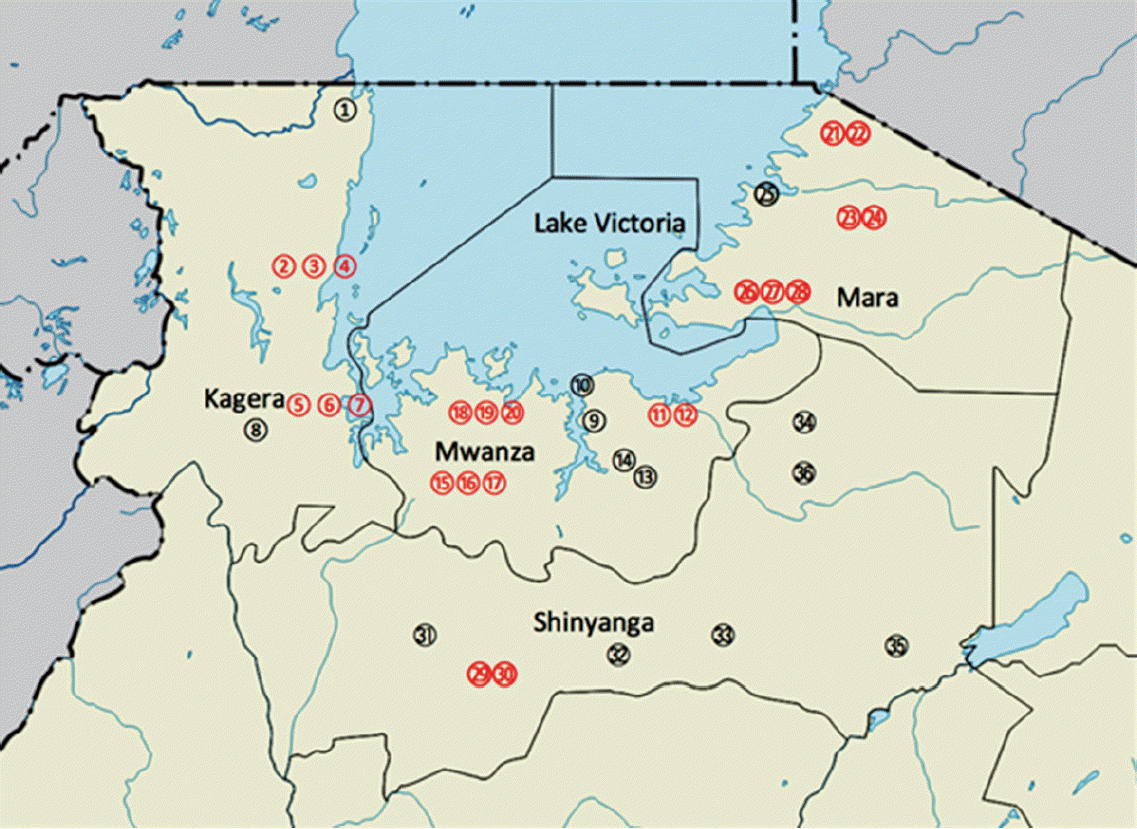
Map of the study areas. Localities in Kagera region (1. Bunena, 2. Kiziramuyaga, 3. Kyenshama, 4. Nyairigamba, 5. Buzirayombo, 6. Bwanga, 7. Bwina, and 8. Runazi), Mwanza region (9. Tumaini, 10. Mazoezi, 11. Lumeji, 12. Nyamikoma, 13. Mwaging’hi, 14. Kigongo, 15. Bugogo, 16. Chibingo, 17. Kasamwa, 18. Nyakalilo, 19. Bungonya, and 20. Busisi), Mara region (21. Gamasara, 22. Ochuna, 23. Marasibora, 24. Minigo, 25. Mwisenge, 26. Guta “A”, 27. Nyamitwebili, and 28. Bulamba), and Shinyanga region (29. Mseki, 30. Bukomela, 31. Masumbwe, 32. Luhumbo, 33. Songwa, 34. Shishiyu, 35. Ngugunu, and 36. Sapiwi).
Specimen collection and examinations
Collection of stool and urine specimens was done at the selected schools. Each child was given 1 stool container and 1 urine container on the first day and asked to bring the urine and stool specimens. The stool samples collected were processed to make duplicate Kato-Katz thick smears [48]. The smears were examined for hookworms and other helminth eggs or larvae within 1 hr after preparation in order to capture hookworm eggs before they hatch. Examination for S. mansoni eggs was done a few minutes later. The egg counts on each of the 2 slides were added together and divided by 2 to get the mean number of egg counts for the 2 slides.
Urine samples were collected after 10.00 a.m. to diagnose S. haematobium based on detection of eggs using the filtration technique [49]. Urine samples were mixed thoroughly and 10 ml were drawn using a syringe and passed through the membrane where eggs were logged. Examination was done on site where detected eggs were counted and expressed as the number of eggs per 10 ml of urine.
Data management and analysis
Data entry was done using Dbase IV (Borland International, Scotts Valley, California, USA), and a double entry system was used for quality control. Data were transferred to STATA version 8 software (Statacorp 2000, College Station, Texas, USA) for analysis. Analysis was done by generation of some frequency tables, cross tabulations, and calculation of the prevalence.
Ethical considerations
The ethical and scientific clearance was obtained from the Medical Research Coordinating Committee of the National Institute for Medical Research before the implementation of the study. The study team visited villages and schools where community leaders and teachers were respectively met and the objectives, procedure, and potential harm and benefits of the study were explained to them. The leaders in turn convened meetings of the community members, and the same was explained to them before the consent to participate in the study was sought. A similar procedure was followed at schools.
Schoolchildren were told that participation to the study would be voluntary and free. Any participant would be free to withdraw from the study at any time of the study period when he or she felt to do so. The decision to refuse or withdraw from the study would have no negative effect on the benefits provided during and after the study.
All subjects who would be found infected with schistosomes and intestinal helminths were treated following standard treatment guidelines using praziquantel and albendazole tablets. Participants were informed that information will be confidentially kept using code numbers instead of names of participants.
RESULTS
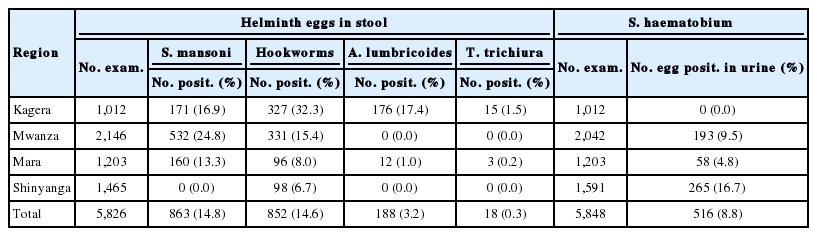
A total of 5,848 schoolchildren from 36 selected schools in the 4 regions of the Lake Victoria basin in Tanzania were recruited for the study (Table 1). The same numbers of girls and boys aged between 7 and 16 years from class 1 to 7 were selected for the study. Intestinal schistosomiasis caused by S. mansoni was prevalent in 3 regions of Kagera, Mwanza, and Mara. The highest prevalence of S. mansoni (24.8%) was recorded in Mwanza region. Hookworms were the only helminths prevalent in all 4 regions. The prevalence of hookworms ranged from 6.7% in Shinyanga to 32.3% in Kagera. Ascariasis and trichuriasis were prevalent in Kagera and Mara regions only. A remarkably high prevalence of ascariasis (17.4%) was found in Kagera region.
Regional mean prevalence of schistosomes and soil-transmitted helminths among schoolchildren in Lake Vitoria Basin, Tanzania
Urogenital schistosomiasis caused by S. haematobium was found in children from 3 regions of Mwanza, Mara, and Shinyanga but not in Kagera. Among the 3 regions, the highest prevalence (16.7%) was recorded in Shinyanga region. The mean prevalence of both schistosomiasis and STHs in all regions ranged from low to moderate ones (Table 1).
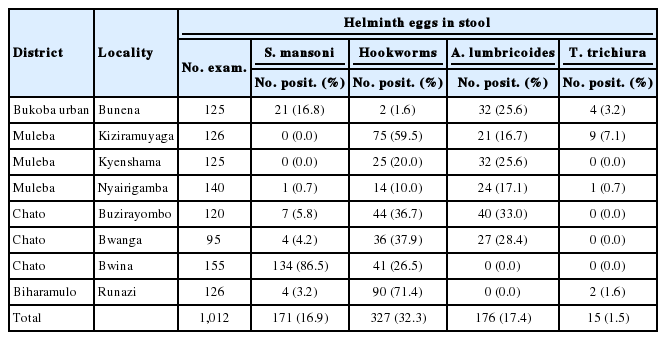
In Kagera region, all the common intestinal helminths, namely S. mansoni, A. lumbricoides, T. trichiura, and hookworm infections were prevalent (Table 2). Bwina Primary School in Chato District recorded an extremely high prevalence of intestinal schistosomiasis of 86.5% followed by Bunena in Bukoba Urban District with a prevalence of 16.8%, and other schools had a prevalence of less than 10%. No S. haematobium positive cases were recorded in Kagera region. Hookworms were prevalent in the Kagera region. The highest prevalence was 71.4% at Runazi School followed by Kiziramuyaga 59.5% and Bwanga 37.9% (Table 2). The prevalence of ascariasis was the highest at Buzirayombo 33.3%, followed by Bwanga 28.4%, Bunena 25.6%, and Kyenshama 25.6% (Table 2). The prevalence of trichuriasis in the region was relatively low with a mean of 1.5% where Kiziramuyaga had the highest prevalence of 7.1%.
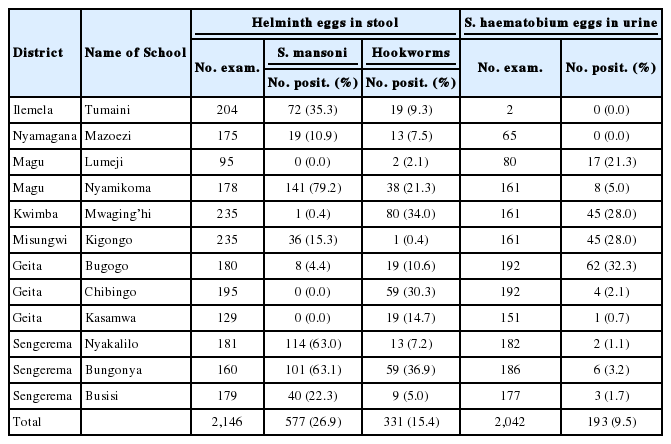
In Mwanza region, 3 helminth species detected included S. mansoni, S. haematobium, and hookworms (Table 3). The highest prevalence of S. mansoni infection was recorded at Nyamikoma Primary School that registered 79.2%, followed by Bungonya 63.1% and Nyakaliro 63.0% (Table 3). Bungonya Primary School had the highest prevalence of hookworms of 36.9% followed by Mwagingh’i 34.0% and Nyamikoma 21.3%. The highest prevalence (32.2%) of urogenital schistosomiasis was at Bugogo Primary School (32.3%), and Mwaging’hi and Kigongo were the second with a prevalence of both 28.0%. Lumeji was the fourth with 21.3% prevalence while the rest of schools had prevalence less than 10% (Table 3).
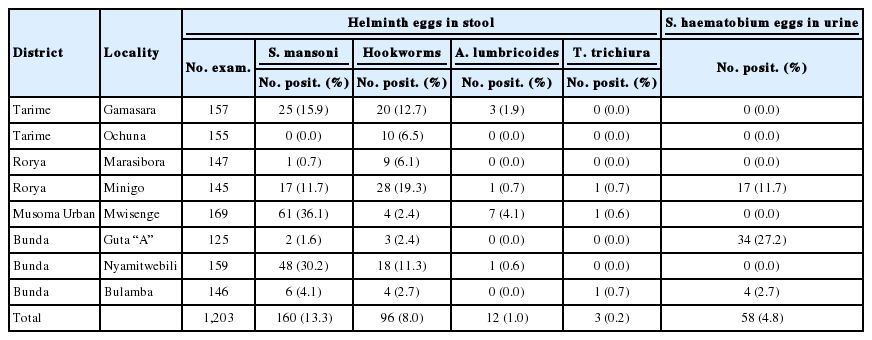
The distribution of schistosomiasis and STHs in Mara region was low relative to other regions (Table 4). S. mansoni was highly prevalent at Mwisenge 36.1%, Nyamitwebili 30.2%, Gamasara 15.9%, and Minigo 11.7%. Other schools had prevalence lower than 10% (Table 4). Urinary schistosomiasis was found in 3 schools only. The prevalence was the highest (27.2%) at Guta “A” School followed by Minigo 11.7% and Bulamba 2.7%. The prevalence of hookworms in Mara region ranged from 2.4% at Mwisenge to 19.3% at Minigo Primary School. The prevalence of trichuriasis was the lowest of all the helminths in region.
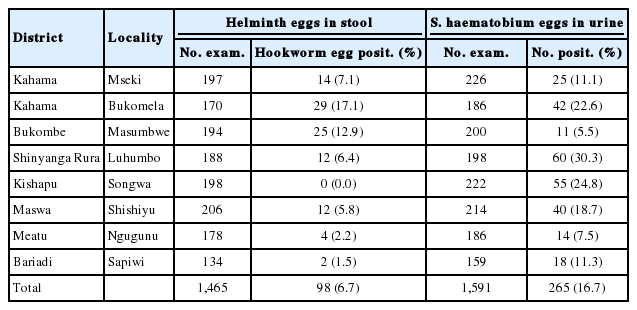
In Shinyanga region, urogenital schistosomiasis was prevalent in all schools studied (Table 5). The highest prevalence (30.3%) was noted at Luhumbo School in Shinyanga Rural District seconded by 24.8% at Songwa Primary School in Kishapu District. Intestinal schistosomiasis was not noted in the whole study areas, whereas the hookworm prevalence was low; ranging from 0% at Songwa School to 17.1% at Bukomela School. No ascariasis nor trichuriasis cases were noted at any of the schools in Shinyanga region.
DISCUSSION
The results showed that generally the prevalence of S. mansoni, S. haematobium, and STHs are considerably high in the study areas. Similar observations were reported from earlier studies in Magu and Sengerema in the same areas [10,50]. However, the prevalence of S. mansoni in Kagera region is very low with the exception of a few pockets in Chato District which is currently in Geita region. This could be attributed to the fact that lake shores in Kagera region are open instead of bays when wind blows make the waves splash the shores making the place an unconduncive habitat for snail intermediate hosts. Moreover, some of the shores in Kagera region are formed by escarpments thus deep and less habitable by the snails. The situation is quite different from shores in Chato District which is a bay thus most of its shores are not susceptible to strong winds. The distribution of S. haematobium and S. mansoni along Lake Victoria is largely related to the distribution of the intermediate hosts [51]. Along the shore of the lake, members of the genus Biomphalaria, the intermediate host for S. mansoni, are common [52,53] with populations living along the lake shores and islands being highly affected by S. mansoni as the risk of infection increases [53,54].
The shores in Chato Districts are shallow; thus very conduncive for Biomphararia snails. This is why the district recorded high prevalence of intestinal schistosomiasis. Apart from the fact that schistosomiasis is focal, the noted high prevalence of the disease in this district could be attributed to the presence of bays that experience less speedy winds and strong waves to the shores making the place unconduncive habitat for snails.
The highest prevalence of S. mansoni at Bwina Primary School in Kagera region could be attributed to the location of the study site. Bwina ward is a thin peninsular with an average width of less than 2 km. As such, most of the area is surrounded by Lake Victoria water that is inhabited by Biomphararia species. Bwanga and Kiziramuyaga localities are close to the lake area that has small bays that are conduncive areas for the snail hosts. The absence of urogenital schistosomiasis in Kagera region could be due to the absence of ponds and streams, and the fact that no paddy cultivation is practiced in this area.
The prevalence of STH infections in Kagera region was relatively higher than that in other regions. This could be due to the difference in natural vegetations and gardens as a result of rain variations. Kagera is sunny and warm most of the year with cool evenings and rains occuring almost every morning from March through May [55]. STHs are highly affected by surface temperature [56], altitude, soil type, and rainfall [57]. This has an implication of the region having the best breeding ground of STHs as well as more people consuming semicooked vegetables that may carry helminth eggs.
In Mwanza region, the distribution of S. mansoni was similar to that of Mara with more happenings close to the lake and S. haematobium on the hinterland. The high prevalence of intestinal and urogenital schistosomisiasis in the study area was a function of the distance from Lake Victoria, the former being more prevalent at localities close to the lake, whilist the latter is more so away from it [52-54]. Moreover, in areas where the prevalence of S. haematobium was high, there was a significant prevalence of hookworms. The findings are in line with other studies in Magu District in Mwanza region [10-12]. The spatial distribution of S. haematobium is in accordance to the presence of ponds and streams as well as the wetness and warmth of the soil that are prerequisites for proliferation of the 2 helminths.
We could not easily establish why there were no Ascaris or Trichuris infections in Mwanza and Shinyanga regions that are situated in the middle of the study area. Similar findings were reported by Mazigo on 1 ward in the region [50]. Difference in soil structure and texture as well as climate among the regions could be the reasons for the difference. Kagera region on the west bordering southern Uganda and Mara region on the west which borders western Kenya have similar prevalence of the STHs as compared to Mwanza and Shinyanga regions [50,54]. The prevalence of hookworm infections, 34% at Mwaging’hi in Kwimba District, and 21.7% at nearby Nyamikoma location in Magu District, was similar to an ealier report in the same area [10]. Despite the fact that Mara region is closer to the western province in Kenya with similar types of STH infections, they differed in prevalence. This study recoded a mean regional prevalence ranging from 1% to 8% whereas studies in Kenya’s Nyanza Province reported the prevalence of 22.9% and 17.9% for A. lumbricoides and T. trichuris, respectively [10,50].
In Mara region, the high prevalence of intestinal schistosomiasis at Mwisenge and Nyamitwebili schools were associated with their being very close to the lake shore. The study team could see children sent to the lake to fetch water. The high prevalence of urogenital shistosomiasis at Guta “A” and Minigo could be ascribed to paddy fields, that are good habitats for the snail intermediate host of S. haematobium, that were enormous in the sourounding areas. In Mara region, the distribution of S. mansoni was similar to that of Mwanza. Moreover, in areas where schistosomiasis was high, there was a significant prevalence of hookworms. This association was reported elsewhere; these findings are in line with that from Magu District in Mwanza region and in western Kenya [10,57,58]. As expected, the prevalence of intestinal schistosomiasis was extremely low in Shinyanga region that is more than 150 km away from the lake but had the highest prevalence of urogenital schistosomiasis.
The results showed that schistosomisasis and 3 STHs infections are co-endemic in Lake Victoria basin in Tanzania with a high probability of polyparasitism in the study participants. The prevalence of S. mansoni, S. haematobium, and STHs ranged from low to moderate in most parts of the study area. Intestinal schistosomiasis was prevalent along the Lake Victoria shores and decreased with distance from it. Conversely, the prevalence of urogenital schistosomiasis increased with distance from the Lake.
From the study findings, we recommend the followings to control schistosomiasis in the study area. First, there is a need for provision of health education to community members. This could be given in a participatory manner so that each member gets engaged in the learning process especially through peer educators. Second, study teams need to conduct regular research in order to control incidence and reinfection rates in the area by carrying out regular treatment exercises. Third, community-based provision of adequate safe water supply and sanitation facilities are imperative. Water supply could be done through construction/drilling of wells at each sub-village, institutions, and health facility.
Acknowledgements
The authors are very grateful to the people of Kagera, Mwanza, Mara, and Shinyanga for their tireless commitment to participate in the study for all 5 years running. Our heartfelt thanks go to the Government of the Republic of Korea through the Korea international Cooperation Agency (KOICA). Good Neighbors International (GNI) funded the study and provided expertise for conduction of the study from the beginning to its logical conclusion. Particular gratitude is extended to the staff of NIMR, Mwanza Research Center, and the entire members of GNI Tanzania Western Chapter for their material, moral, and technical support during the study. We would like to thank all who supported us as laboratory technologists from NIMR Mwanza whose commitment and deligency during all the stages of the study that led this study to a success.
Notes
There is no conflict of interest declared by any of the authors.