Therapeutic potentials of Trichinella spiralis in immune disorders: From allergy to autoimmunity
Article information
Abstract
The incidence of immune system diseases is increasing globally, particularly in developed countries. The hygiene and old friend hypotheses suggest that the decreased incidence of helminth infections in these countries may underlie the rising prevalence of autoimmune, allergic, and inflammatory diseases. The preventive and therapeutic potential of Trichinella spiralis, a helminthic parasite, has been well demonstrated in animal models of immune dysregulation-mediated diseases. This review comprehensively analyze how T. spiralis modulates immune responses across a spectrum of immune dysregulation. We systematically review the key research findings on the effects of T. spiralis infection on immune-related disease. T. spiralis has shown the ability to regulate host immune responses in autoimmune, allergic, and inflammatory disorders, exerting anti-inflammatory effects and restoring immune homeostasis through various immunological pathways. Given its significant immunomodulatory potential, T. spiralis represents a promising candidate for therapeutic interventions against immune-mediated diseases, warranting further molecular investigations and clinical applications.
Introduction
The prevalence of immune disorders driven by aberrant immune responses has significantly increased in modern society compared to the past. This trend is largely attributed to improvements in healthcare and sanitation associated with industrialization, which have reduced the immune system’s for exposure to foreign organisms, including bacteria, fungi, and parasites. Consequently, the development of a stable and well-matured immune system is impeded. Notably, the lack of exposure to infections and allergens during critical periods of immune maturation is closely linked to an underdeveloped regulatory immune system, leading to heightened sensitivity and excessive inflammatory or autoreactive responses to external stimuli, such as allergens and pathogens [1–3]. In recent years, considerable attention has been directed toward understanding the regulatory immune responses of helminth infections and their derivatives against bystander pathogens or antigens. These investigations aim to elucidate helminth-modulated host immune responses and assess their potential in preventing and treating autoimmune, allergic, and inflammatory diseases.
Trichinella spiralis is a parasitic nematode that infects various mammalian hosts, including humans, primarily through the consumption of undercooked or raw meat. The modulation of host immune responses by T. spiralis has been observed in various mouse disease models [4,5]. The immune response to T. spiralis is complex and multifaceted, involving both innate and adaptive immune mechanisms [6–9]. Importantly, T. spiralis have evolved sophisticated strategies to modulate the host’s immune system, enabling long-term survival while minimizing harmful inflammatory responses [10,11]. These immunomodulatory properties have garnered significant interest in immune-related diseases.
Studies have shown that T. spiralis infection plays a crucial role in maintaining homeostasis by modulating immune responses toward a more regulatory or anti-inflammatory state in various immune-mediated disorders. This review aims to provide a comprehensive analysis of the immunomodulatory effects of T. spiralis across various immune-related diseases, including autoimmune [12–14], allergic [15–18], and inflammatory disorders [19,20]. Synthesis of key research findings highlights the mechanisms underlying the influence of T. spiralis on disease progression and explores the preventive and therapeutic potential of T. spiralis-based interventions.
Immunoregulation Effects of Trichinella spiralis Across Disease Contexts
Chronic allergic asthma
T. spiralis infection confers protection against experimentally induced allergic asthma, primarily through the enhancement of regulatory immunity. Park et al. [17] and Kang et al. [21] reported that interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β) induce regulatory T (Treg) cells during chronic infection, as they both suppress pro-inflammatory and allergic T cell responses. T. spiralis effectively suppresses allergic T cell responses and innate immune cell-driven inflammation by increasing the proportions of CD103+ dendritic cells (DCs) and alveolar macrophage—both cells of which exhibit anti-inflammatory properties—alongside Treg cells [15]. Previous studies investigated the regulatory capacity of T. spiralis-driven Treg cells and M2 macrophages by the adoptive cell transfer mice experiment system [21,22]. Their findings revealed that Treg cells or M2 macrophages driven by T. spiralis infection had significantly greater preventive and therapeutic effects against ovalbumin induced allergic asthma than Treg cells or M2 macrophages isolated from uninfected mice. Additionally, T. spiralis-derived substances effectively regulate pulmonary allergic responses. For example, soluble total extracts and excretory-secretory products (Ts-ESPs) of T. spiralis significantly ameliorate allergic asthma by reducing airway hyperresponsiveness, eosinophil infiltration, and T helper 2 (Th2) responses through the stimulation of Treg cell activity [15,23]. T. spiralis 49 kDa and 53 kDa proteins—key components of Ts-ESPs—alleviate allergic symptoms, weight loss, and lung inflammation by modulating the imbalanced T helper 1 (Th1)/Th2 cell ratio [16]. Other key substances in Ts-ESP, such as succinate coenzyme A ligase-β-like protein [24] and cystatin [25,26] play crucial roles in modulating pathophysiology and triggering anti-inflammatory immune networks [24–27].
Overall, T. spiralis exhibits a robust capacity to modulate the host’s respiratory allergic immunity, which can be categorized into 2 key aspects: infection-based prevention and derivative-based treatment. Chronic T. spiralis infection exerts strong preventive effects against respiratory allergic diseases despite the initial surge of Th2 responses during the acute infection phase. Conversely, T. spiralis-derived substances exhibit strong therapeutic efficacy. The preventive and therapeutic effects of infection and its derivatives are primarily mediated through the enhancement of regulatory immune cell responses, including the induction of Treg cells and the promotion of anti-inflammatory phenotypes of DCs and macrophages.
Acute airway inflammatory disorders
The immunomodulatory capacity of T. spiralis extends beyond allergic conditions, influencing responses to acute lung injuries and infections. Pre-existing T. spiralis infection attenuate the severity of respiratory infections caused by Pseudomonas aeruginosa and respiratory syncytial virus (RSV) [28,29]. These protective effects arise primarily from the downregulation of pro-inflammatory cytokines. In P. aeruginosa pneumonia, pulmonary inflammation resulting from neutrophil recruitment and the release of inflammatory mediators, including IL-1β, IL-6, CXCL1, and CXCL2, is significantly improved. This reduction in inflammation is mediated through T. spiralis-stimulated Th2 type responses, which also improve survival rates [28]. Given that RSV causes Th2-dominant lung inflammation, the protective mechanism of T. spiralis against RSV differs from that of P. aeruginosa infection. T. spiralis enhances antibody responses to RSV, mitigates lung inflammation by downregulating NF-κB, and upregulates nuclear factor erythroid 2-related factor 2 protein and antioxidant enzyme, NQO1 [29]. Furthermore, the parasite’s therapeutic potential has been explored in viral infections, including influenza and coronavirus disease 2019. During the early phase of T. spiralis infection, co-infections with influenza A virus X31 strain alleviate influenza-induced lung pathology and promote faster weight recovery. This effect is attributed to suppressed inflammatory cell infiltration in the lungs, although it compromises viral clearance. Notably, once T. spiralis has encysted, no significant reduction in lung pathology is observed, highlighting the importance of infection timing in mitigating influenza-related pathology [30]. T. spiralis also alleviates coronavirus disease 2019-induced hyperactivation of pro-inflammatory Th1 cytokine storms, including TNF-α and IFN-γ, via an IL-10/IL-9 axis dependent mechanism. Additionally, TsESP exert protective effects against severe acute respiratory syndrome coronavirus 2 infection in K18-hACE2 mice. Severe acute respiratory syndrome coronavirus 2-induced histophatological changes in the lung, such as pulmonary hemorrhage, widening of alveolar septa, and variable degrees of lymphocyte infiltration, are alleviated by Ts-ESPs [31,32].
Moreover, T. spiralis is applied in infectious disease management. For instance, T. spiralis muscle larvae ESP have been shown to protect against polymicrobial sepsis by downregulating MyD88 signaling via a mannose receptor interaction [33]. Adult TsESP mitigate cecal ligation and puncture-induced sepsis by downregulating the HMGB1/TLR2/MyD88 signal pathway [34]. A previous study demonstrated that the potential therapeutic effects of T. spiralisrecombinant 53-kDa protein attenuates LPS-induced acute lung injury by promoting M2 macrophage polarization and reducing pyroptosis [35].
Neuroinflammation
Multiple sclerosis (MS) is an autoimmune disorder characterized by immune-mediated destruction of myelin in the central nervous system (CNS) [36–38]. Its pathogenesis is strongly influenced by the activity of myelin-specific autoreactive T cells, particularly the Th1 and Th17 subsets, which drive inflammatory responses within the central nervous system [38–40]. Dysfunctional regularly Treg activity further exacerbates immune dysregulation, as evidenced by the impaired suppressive function of CD4+CD25hi Treg cells in patients with MS [36,41–43].
Experimental autoimmune encephalomyelitis (EAE)—an established animal model for MS—has been instrumental in exploring the potential immunomodulatory effects of T. spiraliralis. Chronic T. spiralis infection ameliorates EAE severity in Dark Agouti rats by modulating immune responses, including the induction of IL-10, a well-known key inhibitory factor in EAE [39], and by a Th2 bias and enhanced Treg cell presence within the CNS [44,45]. Other studies have also demonstrated that T. spiralis infection increases IL-4 and IL-10 levels while reducing IFN-γ and IL-17 production in EAE-induced mice [46,47].
Further insights have been gained from studies utilizing T. spiralis-derived antigens. The protective mechanism of Ts-ESPs against EAE involves the induction of tolerogenic DCs. Specifically, Ts-ESPs prime DCs to suppress the development of Th1 and Th17 cells. In this regard, Ts-ESPs-primed DCs render tolerance, enhancing Treg cell responses while suppressing autoreactive Th1 and Th17 responses in EAE models [46,47].
Beyond MS, T. spiralis also demonstrated therapeutic potential in neuropathic pain models. In an experimental model of vincristine-induced painful peripheral neuropathy, both T. spiralis infection and Ts-ESPs effectively alleviated neuroinflammation by reducing macrophage infiltration around the sciatic nerve and suppressing the production of IL-1β, a key pro-inflammatory cytokine associated with macrophage activation [48].
Type 1 diabetes
Studies on the association between type 1 diabetes (T1D) and T. spiralis remain limited. T1D is a Th1-driven inflammatory disease, and several studies have demonstrated that T. spiralis infection can inhibit T1D development by promoting IL-4 production, thereby inducing Th2 immune responses. This enhanced Th2 cell response mitigates Th1-mediated destruction of insulin-producing pancreatic β-cells, preserving glucose homeostasis [49,50]. Furthermore, T. spiralis infection stimulates the expansion of Tregs, which plays a critical role in suppressing autoreactive Th1 and Th17 cell responses, thereby preventing pancreatic islet destruction [49,50].
These findings suggest that T. spiralis and its antigens can modulate autoimmune and metabolic pathways, offering a promising therapeutic approach for T1D management.
Rheumatoid arthritis
Th17 cells play a crucial role in the pathogenesis and development of rheumatoid arthritis [51–53]. Tregs counteract Th17 cell activity either direct cell-to-cell contact and the secretion of regulatory cytokines, TGF-β and IL-10 [53–55]. Studies have demonstrated that T. spiralis infection or exposure to its ESP promotes Treg cell expansion, leading to inflammation in mouse arthritis models [56,57]. Notably, the programmed death 1 expression on CD4+ T cells is essential for the attenuation of T. spiralis-attenuated arthritis in mice [57]. Upregulated programmed death 1 expression drives inhibitory signaling for self-tolerance, suppression of inflammatory T cell activity, and delayed tissue damage progression [58]. Moreover, recombinant proteins from T. spiralis, such as paramyosin, have been shown to reduce arthritis severity by inhibiting proliferation and inducing the apoptosis of autoreactive CD4+ T cells. This protective effect is mediated through the upregulation of Treg cells and the induction of indoleamine 2,3-dioxygenase in DCs, which collectively underpin the anti-arthritic effects of T. spiralis [14,59]. Additionally, T. spiralis infection inhibits the polarization of M1-type monocytes and macrophages–key contributors to inflammation and bone degradation [60]. By promoting a more tolerogenic environment in both innate and adaptive immune responses, T. spiralis effectively prevents joint damage and severe inflammation.
Inflammatory bowel disease
Inflammatory bowel disease (IBD) is an actively studied disease regarding the preventive and therapeutic effects of T. spiralis. Therefore, the regulatory mechanisms of T. spiralis in IBD are comparatively well understood. Experimental mouse models of IBD, including dextran sulfate sodium (DSS)-induced and trinitrobenzene sulfonic acid-induced colitis of T. spiralis and its derivatives. Despite the opposing immune responses of Th1/Th17-driven inflammation in DSS and Th17-driven inflammation in trinitrobenzene sulfonic acid colitis, T. spiralis infection confers significant protective immunity against both types of colitis. The mechanisms underlying T. spiralis and derivatives IBD involve a combination of enhanced regulatory immune responses [12,20,61–64], modulation of host cell stress responses [65–67], and alterations in gut microbiota [68–71]. Most studies focused on the relationship between T. spiralis and exerted regulatory immunity. During T. spiralis infection, their antigens, including serine protease inhibitors, reduce Th1-mediated inflammatory responses by promoting the production of IL-13 from Th2 and TGF-β from Treg cells [20,61,62] in mouse models of colitis.
Moreover, infection with T. spiralis, its ESPs, and recombinant proteins have been shown to alleviate IBD symptoms and severity by inhibiting pro-inflammatory cytokines (Th1/Th17), promoting the expansion of Th2 and Treg cells [12,72,73] and inducing M2 macrophage polarization [63,74–76]. These anti-inflammatory effects are mediated via the STAT6/PPARγ pathway [76] or the programmed death 1 and nuclear factor erythroid 2-related factor 2 pathways [74,75]. Notably, in DSS colitis models—which are Th1-prone—T. spiralis suppresses Th1 responses by promoting activated Th2 immunity. Recent proteomic analyses identified 13 T. spiralis factors that drive Th2-mediated immune responses [11]. Furthermore, the adoptive transfer of T. spiralis infection- and Ts-ESPs-driven M2 macrophages reduce the severity of DSS-induced colitis [22]. This phenomenon strengthens both innate and adaptive regulatory immunity, shifting inflammatory responses toward anti-inflammatory profiles, inhibiting epithelial and crypt destruction, and preventing mucosal mast cell hyperplasia. While most T. spiralis-IBD animal studies have demonstrated robust preventive effects against chronic infections, Zheng reported that introducing T. spiralis during the acute phase of DSS-induced colitis conferred superior protective benefits compared with pre-exposure to T. spiralis through the suppression of both Th1 and Th2 responses and the upregulation of the regulatory cytokines IL-10 and TGFβ [77].
Additionally, T. spiralis mitigates colitis by reducing endoplasmic reticulum stress [65–67] and oxidative stress in intestinal epithelial cells. In gasdermin D-mediated pyroptosis, reducing excessive inflammatory cell death and maintaining intestinal epithelium integrity [67]. Furthermore, extracellular vesicles and recombinant serine protease inhibitors from T. spiralis prevent colitis by inhibiting pro-inflammatory M1 macrophage polarization [78,79].
Regarding gut microbiota alteration, T. spiralis infection and its recombinant galectin promote a beneficial microbial composition, leading to anti-inflammatory responses. These changes enhance intestinal barrier function, reduce pathogen translocation, and mitigate intestinal inflammation [68–71]. However, the protective effects are dependent on the timing of T. spiralis exposure. During the invasive phase, T. spiralis infective larvae can damage intestinal epithelial junctions [80,81], leading to apoptosis, nuclear pyknosis, and nuclear rupture through the upregulation of IL-1 and the downregulation of regulatory cytokines (IL-10 and TGFβ) and tight junction-associated proteins (ZO-1, CLDN-3, and OCLN) [81].
Metabolic disorders
Metabolic disorders are characterized by imbalances in metabolic processes, including lipid metabolism, and glucose regulation, that disrupts energy homeostasis. These metabolic abnormalities often result in chronic health issues such as obesity, insulin resistance, and fatty liver disease, which further risk of cardiovascular diseases. Several studies have demonstrated an inverse correlation between T. spiralis and metabolic syndromes such as obesity [18,82,83], nonalcoholic fatty liver disease (NAFLD) [19,84], and nonalcoholic steatohepatitis [85] in animal models.
For instance, chronic T. spiralis infection and its secreted products Ts-ESP have shown compelling anti-obesity effects and anti-hepatic steatosis in mouse models of high-fat diet–induced obesity or NAFLD/nonalcoholic steatohepatitis. An underlying functional mechanism of T. spiralis is to reduce intracellular lipid accumulation in both liver and adipose tissue by inhibiting key adipogenic regulators, peroxisome proliferator-activated receptor gamma, CCAAT-enhancer-binding protein alpha and adipocyte protein 2. Moreover, T. spiralis infection and Ts-SEPs effectively suppress obesity-related systemic inflammatory responses and hepatic inflammation, including proinflammatory adipose or hepatic macrophages and T cells, and strengthen metabolic function by promoting M2 macrophages and Treg cells [18,19,82]. Additionally, Ts-ESPs enhanced the activation of brown adipose tissue and lipid metabolism and reduced intestinal dysbiosis, and intestinal barrier permeability by inhibiting the LPS/TLR4 axis of inflammation [83]. Regarding this, Yang et al. [85] reported that Ts-ESPs significantly alleviated hepatic steatosis and inflammation both in vitro and in vivo by inactivating the TLR4/MYD88/NF-κB inflammation pathway and the NLRP3 inflammasome while promoting M2 macrophage polarization, resulting in attenuated lipid accumulation, inflammation, and oxidative stress. More importantly, these anti-inflammatory effects of T. spiralis in both obesity and NAFLD improve glucose tolerance and insulin sensitivity while preventing excessive adipose tissue expansion and LPS translocation into the liver [19,82].
Furthermore, the modulation of gut microbiota toward a beneficial composition, especially, a decreased ratio of Firmicutes to Bacteriodetes and increased abundance of Akkermansiaceae and Rikenellaceae, is another key mechanism. In high-fat diet–fed mice, the Firmicutes/Bacteriodetes is reduced following chronic T. spiralis infection and is closely associated with persistent anti-obesity effects even after the elimination of T. spiralis [84]. Moreover, transplantation of microbiota from T. spiralis infected mice altered the pathogenesis of NAFLD by suppressing the abnormal expression of hepatic lipid-induced inflammatory genes such as TNF-α and IL-1β [19].
Collectively, these findings highlight the multifaceted role of T. spiralis in modulating metabolic dysfunction. Specifically, T. spiralis-derived substances hold potential as novel therapeutic interventions for obesity, type 2 diabetes, and other metabolic syndromes.
Conclusion
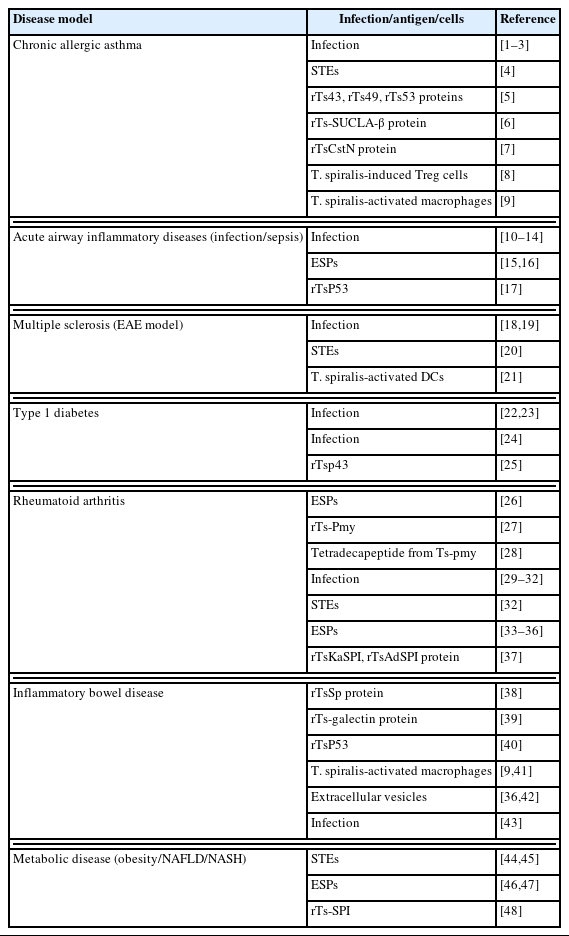
The immunological benefits of T. spiralis infection have been extensively documented through numerous preclinical studies involving various diseases, including allergic asthma, IBD, MS, and diabetes (Table 1) [1–48]. In most immune disorders, T. spiralis infection exerts protective and therapeutic effects by promoting anti-inflammatory cytokine production, activating the Th2 response, and as well as establishing a regulatory immune network comprising Treg cells, M2 macrophages, and tolerogenic DCs (Fig. 1). This T. spiralis-driven modulation of the immune systems supports the long-term survival of T. spiralis by dampening host defenses but also facilitates and demonstrates potential for therapeutic in inflammatory, allergic, and autoimmune diseases.

Mechanisms of Trichinella spiralis-mediated immune regulation across inflammatory contexts. Infection of T. spiralis or its excretory-secretory products (Ts-ESPs) or soluble total extracts (Ts-STEs) modulates immune responses associated with allergic, inflammatory, and autoimmune disorders. Key mechanisms include (1) activation of the polarization of tolerogenic and anti-inflammatory immune cells, such as M2 macrophages, tolerogenic dendritic cells, and regulatory T (Treg) cells, leading to anti-inflammatory or anti-allergic, or anti-autoreactive immune responses; (2) alteration of the intestinal microbiome, restoring a balanced Firmicutes-to-Bacteroidetes ratio, which promotes anti-inflammatory effects, the reduced risk of intestinal and metabolic disorders by enhancing energy metabolism efficiency. Illustration created using Biorender.com. NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis.
Although these findings indicate T. spiralis may have potential in treating refractory or chronic immune diseases, further researches are needed to elucidate the underlying mechanisms and fully explore possible clinical applications. The use of live T. spiralis therapy may be perceived as unappealing and ethically questionable for patients with autoimmune diseases. Therefore, further exploration of T. spiralis-derived molecules is essential, as this approach could pave the way for alternative therapeutic strategies.
Notes
Author contributions
Conceptualization: Yu HS
Data curation: Cho M
Funding acquisition: Yu HS
Software: Cho M
Writing – original draft: Cho M
Writing – review & editing: Yu HS
Conflict of interest
The authors declare no conflict of interest related to this study.
Acknowledgments
This work was supported by the National Research Foundation of Korea (NRF) grant, funded by the Korea government (MSIT) (RS-2024-00347243).