Seroprevalence and risk factors of Toxoplasma gondii infection in pet dogs in Hunan Province, subtropical China
Article information
Abstract
Toxoplasma gondii infections are ubiquitous in both animals and humans. Although seroprevalence data exist for pet dogs across multiple Chinese provinces, limited epidemiological information is available for T. gondii infection in pet dogs in subtropical China’s Hunan Province. We examined T. gondii antibodies in pet dogs from Hunan Province using the indirect hemagglutination test. Logistic regression analyses were used to identify factors associated with T. gondii infection (season, sex, age, breed, and location). The overall seroprevalence was 10.8% (95% confidence interval (CI)=9.0–12.6) (118/1,092), with regional variations ranging from 8.0% (95% CI=4.2–11.8) to 21.1% (95% CI=8.1–34.0). Antibody titers followed a descending distribution: 42.3% (1:64), 30.5% (1:128), 20.3% (1:256), 5.1% (1:512), and 1.7% (1:1,024). The multivariate analysis identified the season (highest in summer: odds ratio=2.0, 95% CI=1.2–3.4) and age (>3 years: odds ratio=2.8, 95% CI=1.5–5.3) as factors independently associated with the outcome (P<0.05). These finding revealed the high seroprevalence of T. gondii infection in pet dogs in Hunan Province, subtropical China, highlighting the risk of zoonotic transmission. Therefore, effective measures should be taken to prevent and control toxoplasmosis in pet dogs in this province.
Toxoplasma gondii, an obligate intracellular protozoan, poses significant clinical risks for immunocompromised individuals and congenital infections [1]. Human transmission primarily occurs through the ingestion of oocyst-contaminated environmental substrates or undercooked meat containing tissue cysts [2]. Despite its global health burden, the prevention of toxoplasmosis remains challenging because of the unavailability of commercial vaccines and the paucity of therapeutic options [3].
T. gondii infection is also highly prevalent in domestic animals, causing substantial economic losses worldwide [4]. Globally, the seroprevalence of T. gondii in dogs varies significantly by region—reflecting differences in climate, husbandry practices, and diagnostic methods. In China, the pet dog population has experienced a rapid expansion, surpassing 52 million in 2023, with an annual growth rate of 2%. A few surveys have indicated that T. gondii infection in pet dogs can cause health problems in these animals and threaten humans [5]. T. gondii oocysts ingested by dogs can pass through the digestive tract and remain infectious [6]. Dogs may indirectly carry these oocysts from contaminated environments [7]. Thus, T. gondii infection in dogs can serve as an indicator of human environmental contamination levels for humans [8]. Recent investigations into the worldwide seroprevalence of T. gondii infection in dogs (including pet dogs) have shown significant variability depending on the geographical region and study design, making comparisons challenging but demonstrating the wide geographical distribution of this parasite [9]. In recent years, several reports of T. gondii infection in pet dogs in China have emerged [10]. However, these studies were mostly published in local Chinese journals, making them inaccessible to foreign scholars. Moreover, limited epidemiological information is available on T. gondii infection in pet dogs in subtropical China’s Hunan Province.
This study aimed to determine the seroprevalence of T. gondii in pet dogs in Hunan Province. The results of this study should provide a basis for the implementation of control strategies against T. gondii infection in pet dogs in this province and elsewhere.
All procedures involving animals in the present study were approved by the Animal Ethics Committee of Hunan Biological and Electromechanical Polytechnic (approval No. 202211). A total of 1,092 blood samples were collected from pet dogs visiting 24 veterinary clinics (6 per zone) across 4 geographical zones (east, south, west, and north) in Hunan Province, China (Table 1). The inclusion criteria were complete records of season, sex, age, breed, and location; dogs with incomplete records or recent antiparasitic treatment (2 weeks) were excluded. One blood sample was collected from each dog, and all blood samples were labeled individually and cooled during transport to the laboratory at Hunan Biological and Electromechanical Polytechnic (Changsha, Hunan Province). There, these samples were centrifuged at 1,000 g for 10 min, after which the supernatant serum was obtained, frozen, and stored at −20°C until use.
Serum samples were analyzed using a validated indirect hemagglutination test kit (NY/T 573-2002, Lanzhou Veterinary Research Institute, Lanzhou, China) as per the manufacturer’s recommendations and previous descriptions [11,12]. This kit has been extensively used for detecting specific antibodies to T. gondii in dogs in China for many years [13]. Samples with titers of ≥1:64 were considered positive.
Data were analyzed with PASW Statistics version 18 (SPSS Inc., Chicago, IL, USA). Continuous variables were presented as mean values with standard deviations for normally distributed data or median values with interquartile ranges for non-normally distributed data. Categorical variables were presented as frequencies with percentages. Continuous data were compared among the groups using the Kruskal-Wallis test or the one-way ANOVA for non-normally or normally distributed data, respectively. Categorical data were compared among groups using chi-square tests. The measure of association in this study was the odds ratio with its 95% confidence interval (CI). Factors associated with T. gondii infection were identified using univariate and multivariate logistic regression analyses. Factors with P<0.2 in the univariate analysis were selected for the multivariate analysis model, which identified factors independently associated with T. gondii infection. The best model was judged using Fisher’s scoring algorithm. A P-value of <0.05 was considered statistically significant.
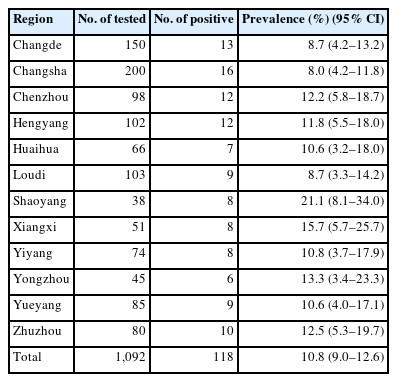
Antibodies against T. gondii were detected in 10.8% (95% CI=9.0–12.6) (118/1,092) of the pet dogs, with titers of 1:64 in 50, 1:128 in 36, 1:256 in 24, 1:512 in 6, and 1:1,024 in 2. This finding indicates that T. gondii infection is widespread among pet dogs in Hunan Province. This finding is similar to that of a recent study showing that the seroprevalence of T. gondii in cats in Hunan Province is 6.4%, which is higher than that in most other provinces of China [14]. The seroprevalence of T. gondii in pet dogs from different regions ranged from 8.0% (95% CI=4.2–11.8) to 21.1% (95% CI=8.1–34.0) (Table 1), with statistically significant differences (P<0.01). The seroprevalence of T. gondii in pet dogs from 7 out of the 12 representative administrative regions in Hunan Province exceeded 10.8% (95% CI=9.0–12.6) (the average value), with the highest seroprevalence (21.1%) (95% CI=8.1–34.0) observed in Shaoyang (Table 1). One plausible reason for this finding is that Shaoyang harbors a relatively higher density of stray cats (the cat is the definitive host of T. gondii). In this study, the seroprevalence of T. gondii in pet dogs was higher than that in Henan (0.8%) (P<0.05) [15] and Liaoning Provinces (10.0%) (P<0.05) [16] but lower than that in Anhui (18.6%) (P<0.05) [17], Shandong (15.6%) (P<0.05) [18], and Yunnan (21.6%) (P>0.01) Provinces [13]. These differences may be attributed to detection methods, geographical environments, overall sample sizes, and animal husbandry practices.
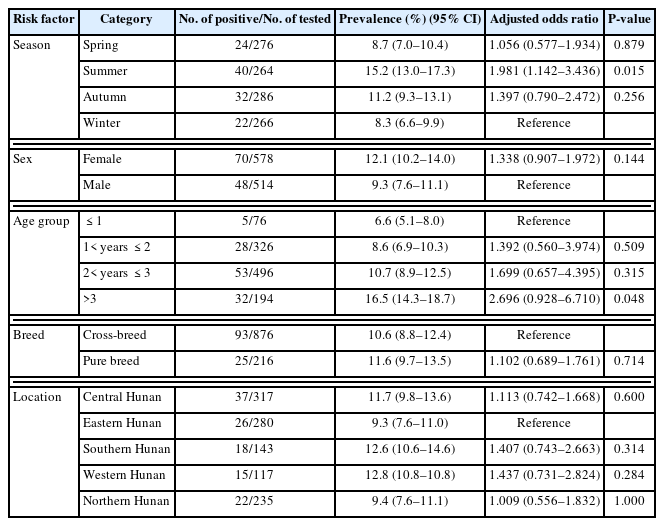
The T. gondii seroprevalence in pet dogs was higher in summer (15.2%) (95% CI=13.0–17.3), followed by autumn (11.2%) (95% CI=9.3–13.1), and lower in spring (8.7%) (95% CI=7.0–10.4) and winter (8.3%) (95% CI=6.6–9.9) (Table 2). This result suggests that T. gondii is prevalent throughout the year, with peaks in summer and autumn. Our multivariate analysis revealed that the odds of being seropositive in summer was 2 times higher than that in winter (P<0.05). This seasonal pattern likely reflects the influence of climatic conditions on oocyst survival and transmission dynamics. Hunan Province has a subtropical moist monsoon climate, with an average annual temperature of 16°C–18°C and high humidity in summer and autumn, which is conducive to oocyst survival [19]. The peaks in T. gondii seroprevalence in summer and autumn suggest that controlling toxoplasmosis during these 2 seasons is critical. The seroprevalence of T. gondii in pet dogs aged >3 years (16.5%; 95% CI=14.3–18.7) was higher than that in pet dogs aged ≤1 year (8.6%; 95% CI=6.9–10.3) (Table 2), and these differences were statistically significant (P<0.05). Our multivariate analysis also showed that dogs aged >3 years had 3 times the odds of seropositivity compared to those aged ≤1 year. This suggests cumulative exposure over time, as older dogs have had more opportunities to encounter contaminated environments or ingest infected prey.
Multivariate analysis of risk factors and seroprevalence of Toxoplasma gondii in pet cats in Hunan Province, subtropical China
The high seroprevalence of T. gondii in pet dogs in Hunan Province, along with the identified season and age as risk factors, can be further interpreted in the context of environmental and host-related factors. The subtropical moist monsoon climate of Hunan Province plays a crucial role as an environmental factor. The warm and humid conditions in summer and autumn not only promote the survival and development of T. gondii oocysts in the environment but also facilitate the activities of potential intermediate hosts, such as rodents and birds. These intermediate hosts can carry and spread T. gondii, increasing the risk of pet dog exposure. For example, rodents may contaminate food and water sources with T. gondii oocysts, and pet dogs that come into contact with these contaminated sources are more predisposed to infection.
Regarding host-related factors, the age-dependent increase in seroprevalence can be explained by the immune status and exposure history of pet dogs. Younger dogs, especially those less than a year old, may have relatively immature immune systems; however, they also have less long-term exposure to the parasite. As dogs age, their immune systems mature; however, they accumulate exposure experiences over time. Additionally, older dogs may have different behavioral patterns than younger ones. They may be more active outdoors, exploring more areas, and coming into contact with various potential sources of infection, further increasing their T. gondii infection risk.
Pet dogs are the most common human companion animals, yet they can carry zoonotic pathogens such as T. gondii, raising public health concerns, as dogs can mechanically transmit T. gondii oocysts to humans via contaminated fur, paws, or oral contact. Although dogs are not definitive hosts, their role as sentinels for environmental oocyst contamination is well-documented. Some surveys have demonstrated that owners of pet dogs have a significantly higher T. gondii seroprevalence than people who do not own such animals [20]. Pet dogs infected with T. gondii may exhibit similar clinical manifestations to canine distemper, making misdiagnosis common in some pet hospitals. Currently, there are approximately 4 million pet dogs in Hunan Province, and most pet hospitals do not offer T. gondii infection detection services for pet dogs, indicating a potential threat to public health in Hunan Province, one of China’s renowned tourist destinations.
Nevertheless, this study has several limitations that should be acknowledged. First, the cross-sectional design employed prohibits temporal trend analyses. Second, there was inherent sampling bias toward urban veterinary clinics, underrepresenting tendencies in rural areas. Third, there was no genetic typing to characterize T. gondii strains. In view of these limitations, future studies should employ longitudinal study designs and molecular methods across diverse habitats.
In conclusion, this study demonstrates a high seroprevalence of T. gondii infection in pet dogs in subtropical China’s Hunan Province, with season and age identified as independent risk factors for the infection. However, this serious situation has received little attention in the past. Therefore, effective measures should be taken to prevent and control toxoplasmosis in pet dogs in this province. These measures could include strengthening environmental sanitation management (especially during summer and autumn), promoting regular T. gondii testing for pet dogs (especially older ones), and enhancing public awareness of the zoonotic risks of T. gondii infection in pet dogs.
Notes
Author contributions
Conceptualization: Wen XX, Liu Z, Xu PY
Data curation: Wen XX
Formal analysis: Wen XX
Funding acquisition: Xu PY
Investigation: Wen XX
Methodology: Wen XX
Project administration: Liu Z, Xu PY
Resources: Xu PY
Software: Xu PY
Supervision: Liu Z, Xu PY
Validation: Liu Z, Xu PY
Visualization: Liu Z, Xu PY
Writing – original draft: Wen XX
Writing – review & editing: Xu PY
Conflict of interest
The authors declare no conflict of interest related to this study.
Acknowledgments
This study was supported by the Scientific Research Project of Hunan Province Department of Education (grant No. 23B1002) and Hunan Provincial Natural Science Foundation of China (2025JJ80377).