Recurrent Hepatic Alveolar Echinococcosis: Report of The First Case in Korea with Unproven Infection Route
Article information
Abstract
Human alveolar echinococcosis (AE), a hepatic disorder that resembles liver cancer, is a highly aggressive and lethal zoonotic infection caused by the larval stage of the fox tapeworm, Echinococcus multilocularis. E. multilocularis is widely distributed in the northern hemisphere; the disease-endemic area stretches from north America through Europe to central and east Asia, including northern parts of Japan, but it has not been reported in Korea. Herein, we represent a first case of AE in Korea. A 41-year-old woman was found to have a large liver mass on routine medical examination. The excised mass showed multinodular, necrotic, and spongiform appearance with small irregular pseudocystic spaces. Microscopically, the mass was composed of chronic granulomatous inflammation with extensive coagulation necrosis and parasite-like structure, which was revealed as parasitic vesicles and laminated layer delineated by periodic acid-Schiff (PAS) stain. Clinical and histologic features were consistent with AE. After 8 years, a new liver mass and multiple metastatic pulmonary nodules were found and the recurred mass showed similar histologic features to the initial mass. She had never visited endemic areas of AE, and thus the exact infection route is unclear.
INTRODUCTION
Echinococcus multilocularis is the fox tapeworm and the most pathogenic one among the 4 zoonotic species of the genus Echinococcus, and thus E. multilocularis can cause an infection resulting in a potentially fatal chronic liver infection called human alveolar echinococcosis (AE) [1]. E. multilocularis has an extensive geographic range, mainly across the northern hemisphere, including endemic regions in central Europe, most of northern and central Eurasia, extending eastward to Japan and parts of North America [1,2]. Foxes and other canids are the natural definitive hosts of E. multilocularis. The intermediate hosts, mostly rodents and ungulates including humans, are infected by accidental ingestion of eggs shed in the feces of the definitive hosts [3]. The metacestodes go through the small intestine and mostly reach the liver, but also other organs such as the lung and brain. The definitive hosts are infected by ingestion of parasitized organs of the intermediate hosts.
Human AE is a potentially fatal, chronically progressive hepatic infection with E. multilocularis, that is characterized by a long asymptomatic incubation period during which an invasive tumor-like multi-vesicular and exogenously budding mass-like lesion is developed. The parasitic lesion composes of dense granulomatous infiltration and microcalcifications microscopically, and eventually necrotic cavitation may occur [1]. In Korea, AE has never been reported, and herein, we report the first written case of human hepatic AE in a southeastern Korean district, Gimhae-si (city).
CASE RECORD
A 41-year-old woman residing in Gimhae-si, Gyeongsangnam-do, Korea transferred to Dong-A University Hospital on 5 April 2002 for evaluation of the known hepatic mass. She had no significant history of past illness, except heavy alcohol consumption. She denied any contact history with domestic animals or pets as well as livestock, and has not visited zoo and never traveled abroad. On 17 September 2001, she visited a regional hospital for check-up her epigastric pain. Routine radiologic examinations revealed a hepatic mass, and the pathologic findings of needle biopsied mass were described as coagulation necrosis.
At Dong-A University Hospital, the liver showed an 8 cm sized ill-defined mass with 1.5 cm and 3 cm sized satellite nodules at the left lateral segment of the liver without lymph node enlargement on CT imaging. Contrast enhanced CT scan demonstrated low attenuated mass (Fig. 1A, B) and no or poor enhancement after contrast injection. These radiologic findings suggested an inconclusive hepatic tumor with necrosis. Although non-neoplastic mass was considered, malignancy such as cholangiocarcinoma should be ruled out radiologically. Laboratory data from routine CBC, chemistry, serology, electrolyte profiles, hormone analysis of blood, tumor markers (α-fetoprotein, carcinoembryonic antigen, and CA 19-9), and urine analysis were all within normal limits. To rule out any possibility of malignancy, left hepatectomy was done. During operation, frozen section made by the biopsied hepatic mass revealed necrotizing granulomatous inflammation. The lobectomized liver showed a relatively demarcated mass, 11 cm in greatest dimension and abutted several nodular masses of 1-3 cm in diameter, which showed necrosis on cut surface, grossly. Histologically, extensive coagulation necrosis, granulomas, and dispersed acellular membranous shells within cystic spaces were observed. These gross and histologic findings corresponded with a parasitic infection of the liver, but identification of the specific parasite species was not processed. She was discharged without complications.
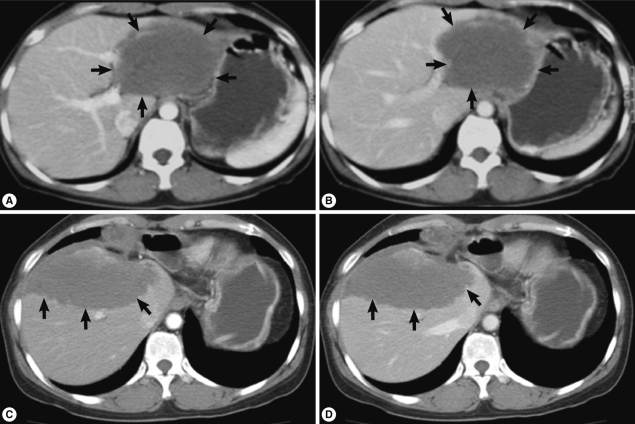
Contrast enhanced abdominal CT scan (A and B, imaging at 1st operation; C and D, imaging at 2nd operation; A and C, arterial phase; B and D, portal phase). CT reveals a low attenuated huge mass (arrows) with little difference between arterial and portal phases.
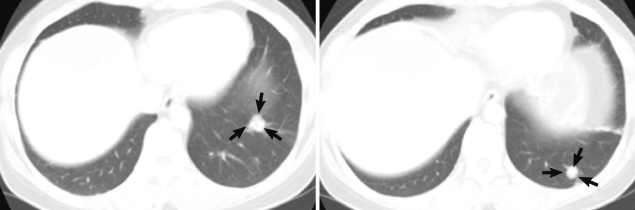
During follow-up, on 19 July 2005, a new nodular mass, 4.5×3.0 cm was observed in S5/8 segment of post-hepatectomized liver on CT imaging, and a needle biopsy of the mass showed coagulation necrosis. The hepatic mass was checked to be enlarged to 6.3×4.5 cm and several small nodules in both basal lungs on 7 August 2007. The hepatic mass showed extensive necrosis, and the pulmonary nodules represented calcification and focally impinged air, suggesting calcified granuloma rather than metastatic malignancy. On 7 May 2010, the hepatic mass was more enlarged, up to 10 cm in diameter and right supradiaphragmatic lymph node enlargement was found on contrast enhanced CT scan. Imaging study showed the same features of the previous mass (Fig. 1C, D). On the lung setting of chest CT scan, relatively well defined small nodules were seen in both lower lobes of the lung, suggestive for calcified granulomas, and metastasis, even less likely (Fig. 2). The eosinophilic count was 8.9% (reference value: 0-7%), total IgE was 1,944 kU/L (reference value: 0-200 kU/L), and parasitological studies were all negative for helminths, including Ascaris lumbricoides, Trichuris trichiura, Enterobius vermicularis, hookworms, and protozoa.
A second operation, including a partial anterior resection, was done but the hepatic mass was not completely removed. The resected liver mass, measuring 16.2×9.5×4.3 cm was rimmed by raggedy, thickened, fibrotic or myxoid Glisson's capsule, focally adhered with fat tissues, and underlying parenchyme showed total necrosis in greenish yellow color, and normal liver was not present. The cut surfaces on serial sections were spongy, with mostly small and occasionally large cystic spaces (Fig. 3). The cystic spaces were empty or filled with mucoid exudates, and inner surface was rimmed by more yellow wall. Between the cysts, yellowish grey rather solid tissues were occupied. Any intact parasite body was not identified. A fragment of the normal liver, 1.5×0.8 cm was separately submitted. Microscopically, the mass showed diffuse and extensive necrosis with totally effaced liver tissues. Among necrosis, numerous variable-sized multilocular cysts with discernible lining were observed. The cysts contained ribbon-like eosinophilic laminated layers, characteristic for E. multilocularis [4], which appeared more strongly red by periodic acid-Schiff (PAS) stain (Fig. 4). Occasionally, fine calcifications were identified between cystic areas. Chronic granulomatous inflammation, composing of proliferating epithelioid cells surrounding the organisms, and infiltration of lymphoplasma cells, a few eosinophils, and giant cells were observed at the periphery of the mass (Fig. 5).
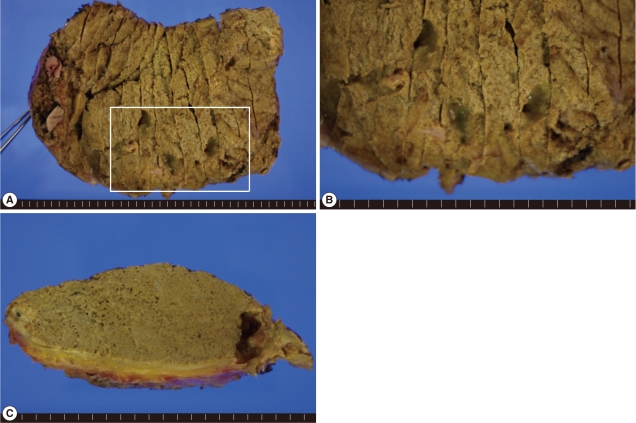
Gross findings of the liver mass (B, higher magnification of the white box of A). External (A and B) and cut (C) surfaces show greenish yellow colored necrotic tissues with mostly microcystic and sometimes large cystic spaces. Glisson's capsule is thickened, showing fibrosis. Grossly normal liver tissues are not seen.
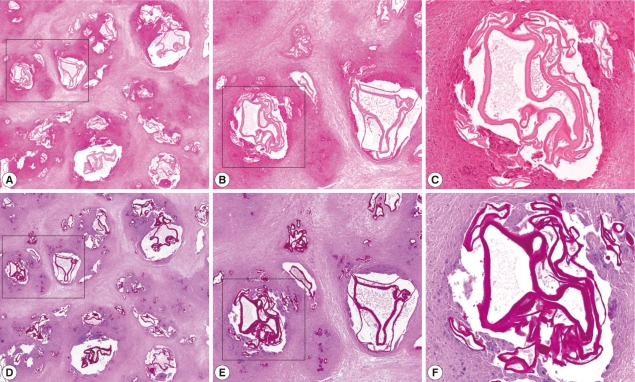
Microscopic findings of the liver mass (A-C, H-E stain; D-F, PAS stain; B and E, higher magnification of the black box of A and D; C and F, higher magnification of the white box of B and E). The mass shows diffuse and extensive necrosis with variable-sized multilocular cystic spaces which includes strong PAS positive ribbon-like and lamellated structures consistent with AE.
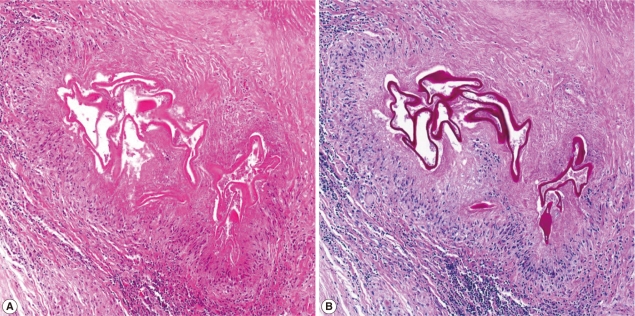
Microscopic findings of the periphery of the liver mass (A, H-E stain; B, PAS stain). The outer portion of necrosis shows chronic granulomatous inflammation with microcystic organisms.
We could approach this unusual hepatic mass, mimicking clinical features of malignancy after consultation to Professor Jong-Yil Chai, Department of Parasitology and Tropical Medicine, Seoul National University College of Medicine, Seoul, Korea. We concluded that these findings corresponded well with hepatic alveolar echinococcosis although Korea is not an endemic area for this cestode disease. She was discharged after the second operation without comlpication. During about 14 months of follow-up period, she has taken 800 mg of albendazole, orally in 2 divided doses, without any symptom. Follow-up radiologic tests showed unremarkable changes of the remaining mass on the remnant liver, and slightly decreased size of pulmonary nodules.
DISCUSSION
AE is restricted to transmission in the northern hemisphere as mentioned. Although, Hokkaido of Japan's northmost island is known as an endemic area [1,3] and in these days, the endemic areas have expanded, in Korea, human AE is not notified to our knowledge. Furthermore, in non-endemic areas, AE is enough to be confused with malignant tumor and misdiagnosed.
We experienced an unusual clinical case, complaining of a hepatic mass with histological features of non-neoplastic and suggestive of a parasite infection and continuous clinical suspicion of malignant tumor for 10 years. Even she has been residing in Gimhae city which is a recently developed and industrialized area, but was a rural community, she denied any history of contact with domestic animals or pets as well as livestock and has been never visited other countries. In this regard, it is strongly suggested that a possible infection route from imported animals may be underlaid. Concerning a literature review of human cestode infections in Korea, Min [5] recognized that most human hydatidosis cases have been imported ones, from outside of Korea, except 1 possible indigenous case [6], and they were all caused by cystic hydatidosis (CE) due to Echinococcus granulosus, not AE.
AE is rare but fatal. It has a risk of metastasis in advanced stages, usually resulting in secondary lesions in the lung or brain. A fatal outcome may occur in over 95% of untreated patients within a 10-year period following diagnosis [1]. When a slowly growing tumor, visualized by imaging techniques, is detected, a case is verified if at least 2 of the following 4 parameters are fulfilled, according to the WHO recommendations: 1) Typical organ lesions detected by imaging techniques, e.g., abdominal ultrasound, CT, and magnetic resonance imaging, 2) Detection of E. multilocularis specific serum antibodies by serotests, 3) Histopathology compatible with AE, 4) Detection of E. multilocularis nucleic acid in a clinical specimen [2,7]. CT imaging of slow growing and recurred mass in this case displayed ill-defined hypodense mass with poor or no enhancement after contrast injection and no differences on arterial and portal phase, which is characteristic for hepatic AE [8]. During the follow-up period of our case, multiple basal nodules at both lungs appeared, showing pulmonary extension. However, it is sometimes difficult to rule out other parasitic infections, pathologically and clinically, especially in non-endemic areas. Multiple cysts, mostly empty and histologically lamellated membrane along the inner side of the cysts with characteristic PAS (+) stain, are compatible with AE [2,4,8].
Moreover, supradiaphragmatic lymph node involvement on CT imaging occurred in our case. Lymphatic spread has been hypothesized to constitute a route for dissemination [9,10], however, the actual human involvement of lymph nodes in AE has not been frequently reported [10]. In our case, lymph node biopsy with studies of AE was not done. We had not performed serologic and molecular studies for detection of E. multicularis-specific serum antibodies and nucleic acids until just prior to publication of this paper. However, these studies were subsequently performed and the results will be published elsewhere. In endemic areas, AE is considered the most serious parasitic zoonosis and AE incidence may increase in the future though the incidence is different among regions. Thus, molecular and immunological technology for detection of humans and animals infected with E. multicularis as well as integrated control measures have been advanced, centering around Japan [11].
Since 1996, guidelines for the treatment of human AE are suggested and documented as a manual [11] and have recently been updated by WHO-IWGE [7]. The rules have been set: radical surgery is the first choice in all cases suitable for total resection of the lesion; benzimidazoles as anthelmintic drugs are mandatory in all patients, temporarily after complete resection of the lesions, and for life in all other cases [2,7,12]. Interventional procedures should be preferred to palliative surgery whenever possible. In our patient, 2 times radical operations were proceeded because hepatic malignancy could not continuously excluded. After the 2nd operation, albendazole was recommended. Albendazole has an efficacy similar to mebendazole, the first successfully used benzimidazole derivative [13] together with some pharmacokinetic advantages [14]. She has been visiting regularly for radiologic follow-up, but till now no remarkable changes have been reported.
Here, we report a case of human AE, representing unusual hepatic mass on clinical examination which showed necrosis with numerous sterile cysts and granulomas on pathologic examination, in a non-endemic area with uncertain infection route.