Influencing Factors for Cure of Clonorchiasis by Praziquantel Therapy: Infection Burden and CYP3A5 Gene Polymorphism
Article information
Abstract
Chemotherapy of clonorchiasis with praziquantel (PZQ) is effective but about 15% of treated cases have been reported uncured. The present study investigated correlation of single nucleotide polymorphisms (SNPs) of the cytochrome P450 gene, CYP3A5 and cure of clonorchiasis. A total of 346 egg passing residents were subjected and treated by 3 doses of 25 mg/kg PZQ. Reexamination recognized 33 (9.5%) uncured and 313 cured. Numbers of eggs per gram of feces (EPGs) before treatment were significantly lower in the cured group than in the uncured group (2,011.2±3,600.0 vs 4,998.5±7,012.0, P<0.001). DNAs of the subjects were screened for SNPs at 7 locations of CYP3A5 using PCR. In the uncured group, the SNP frequencies at g.-20555G>A and g.27526C>T of CYP3A5 were 15.2% and 9.1% while those were 3.8% and 1.0%, respectively, in the cured group. The cure rate was significantly lower in the cases with SNP at g.27526C>T and EPGs≥1,000. In conclusion, EPGs and SNPs of CYP3A5 are factors which influence cure of clonorchiasis by PZQ therapy. It is strongly suggested to recommend 2-day medication for individuals with high EPGs≥1,000.
INTRODUCTION
Clonorchiasis is a liver fluke disease caused by Clonorchis sinensis in East Asia. It is estimated that about 30 millions of infected population live in endemic areas [1]. Its prevalence is still high in Korea along endemic riversides although praziquantel (PZQ) has been widely used [2]. The last 7th national survey revealed 2.9% prevalence of general population in 2004 in Korea.
PZQ is known to be very effective and the drug of choice against clonorchiasis by 3 divided doses, but a small part of medicated patients, 14-17% of them, are not completely cured [3,4]. This incomplete cure is regarded as not a drug resistance but an outcome by individual variation of PZQ pharmacokinetics [5]. Because of rapid absorption and short half-life in human body, PZQ is recommended to use 3 times for medication of liver fluke diseases. Its cure rate for schistosomiasis is also known to be 60% to 80%, and the blood concentration of PZQ varies remarkably by individuals [6]. Therefore, individual variation of pharmacokinetics of PZQ may be important for the medication efficacy and cure of fluke infection.
Cytochrome P450 (CYP450) is a microsomal enzyme complex, which metabolizes endogenous or exogenous compounds, including drugs and toxic chemicals, leading to their easy elimination from human body. Two major isoenzymes, CYP2B1 and CYP3A5, are mainly involved in hydroxylation of PZQ in the liver [6]. Mainly the hydroxy-PZQ in bile keeps the cidal activity on the liver fluke [5]. Among several isoenzyme genes, CYP3A5 has been known to be highly polymorphic and variable by races [7,8]. Polymorphisms of CYP3A5 are suggested to contribute to individual and interracial differences in CYP3A5-dependent drug metabolism [8].
In this context, a hypothesis was proposed that cure of clonorchiasis by PZQ is correlated with pharmacokinetic variation which is determined by genetic polymorphisms of CYP450. The present study investigated the correlation between cure of clonorchiasis by PZQ and genetic variation of CYP3A5.
MATERIALS AND METHODS
Subjected population
A total of 491 residents were recruited at a village in Heilongjiang, China, where clonorchiasis was hyperendemic [9]. They were 253 males and 238 females, age range 9-82 and median 40, and most of them were included partly in a sonographic study [10]. They were examined of their feces for eggs of C. sinensis and their blood samples were collected in September 2004. Of them, 346 were egg positive and medicated with PZQ 25 mg/kg, ×3. They were reexamined 3 weeks after medication and divided into 2 groups, cured or uncured. The residents included in this study were informed and agreed with this study by oral communication.
Fecal examination
The fecal specimens were examined by single smear of the Kato-Katz method [11]. The number of eggs on a smear was counted to estimate numbers of eggs per gram of feces (EPGs).
Single nucleotide polymorphisms (SNPs) of CYP3A5
Three drops of blood were spotted on filter paper and dried at room temperature. The blood spots were put into a 2-ml tube with a screw cap, and dissolved in 200 µl of 5% Chelex-100 resin (Bio-Rad, Hercules, California, USA). Elution at 60℃ for 30 min and boiling at 100℃ for 30 min were carried out, and DNAs were obtained from the supernatant after centrifugation. The tubes were subsequently centrifuged again for 10 min at high speed to separate the surface layer in which the DNA can be found. The DNAs were stored at -70℃ and used for the analysis.
Total 7 primer pairs were designed using known sequences within and upstream of CYP3A5 subfamily as previously described (Table 1) [12,13]. Individual DNAs were used for templates and amplified with the primers by PCR under condition of initial denaturation at 94℃ 5 min, 94℃ 30 sec, 55-60℃ 30 sec, and 72℃ 1 min for 35 cycles, and final extension at 72℃ for 10 min. The amplified fragments were sequenced by capillary electrophoresis method using an automatic sequencer, ABI 3730 DNA analyzer (Applied Biosystems, Branchburg, New Jersey, USA). The sequences were analyzed of SNPs using the program of DNASTAR (DNAStar Inc, Madison, Wisconsin, USA).
Statistical analysis
The program SPSS (version 12.0) was used for statistical analysis. The data were summarized as mean±standard deviation or percentage proportion. Statistical significance of age and EPGs between groups was checked by Student's t-test and that of gender, variation of SNPs was by chi-square analysis. Multiple regression analysis was also applied to analyze the impact of age, gender, EPGs, and SNPs on cure of clonorchiasis. P values less than 0.05 were accepted as significant.
RESULTS
Primary fecal examination of the subjects
Of the 491 subjects, 346 were egg positive of C. sinensis (Table 2). Their mean EPGs was 2,296.2±4,129.5, and 41.5% of them were under EPG 100, 27.5% 100-999, and 31%≥1,000.
The 346 egg positive subjects were examined again 3 weeks after PZQ medication by the same method. Of them, 313 (90.5%) were converted to negative and 33 were still positive (Table 3). Age and gender differences were not significant between the cured and uncured groups, but EPGs, 2,011.2±3,600.0 in cured and 4,998.5±7,012.0 in uncured, differed significantly (P<0.001).
SNP frequency of CYP3A5
Among the 7 sites of the CYP3A5 gene: g.-20619T>G, g.-20555G>A, g.-6177A>G, g.-795T>A, g.19386G>A, g.27526C>T, and g.31611T>C, g.-20555G>A showed the most frequent variation, 17 (4.9%) of 346, and the second was g.-6177A>G, 3.5%. The frequencies were 1.7%-2.3% in other sites (Table 4).
SNPs of CYP3A5 and cure rates
Significant difference of SNP frequency between the cured and uncured groups was recognized in location of g.-20555G>A (Po=0.016) and g.27526C>T (P=0.013). SNP variant of g.-20555G>A was 3.8% in cured group but 15.2% in uncured group, and that of g.27526C>T was 1.0% and 9.1%, respectively (Table 4). No significant difference was found in other locations (Table 4).
Cure rates by EPGs
Cure rates were significantly correlated with EPGs. In the group of EPGs less than 100, all of 59 (100%) treated subjects were cured while 123 (91.1%) and 131 (86.2%) were cured in groups of EPGs of 100-999 and ≥1,000, respectively (P=0.009).
Cure rates by SNP variation in groups of EPGs
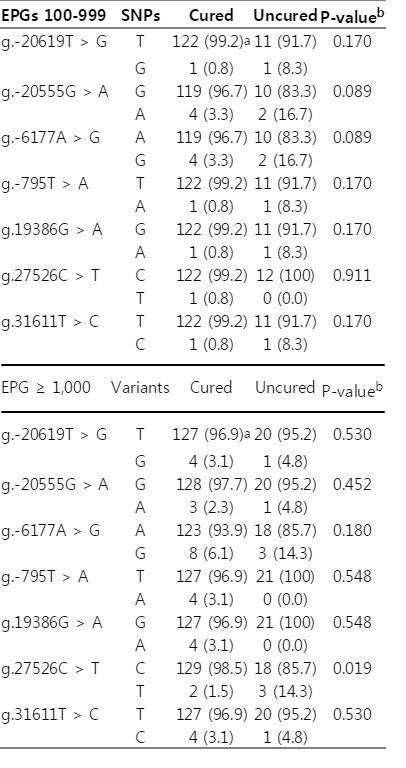
Cure rates were compared by the 7 allelic locations of SNP variation by EPGs. The SNP frequencies of all locations showed no significant differences of cure rates in the group of EPGs 100-999. However, g.27526C>T variants revealed a significantly low cure rate in the group of EPGs ≥1,000 (Table 5).
Multiple regression analysis
All of observed variables were reviewed by multiple regression analysis (Table 6). Age and sex were not significant but EPGs (P=0.004) and SNPs at g.27526C>T (P=0.001) were significant with cure of clonorchiasis.
DISCUSSION
The present study investigated the hypothesis that cure of clonorchiasis by PZQ was determined by genetic polymorphism of CYP3A5. The hypothesis was accepted because the present findings confirmed that SNPs of g.27526C>T and EPGs≥1,000 were significantly correlated.
When PZQ is introduced into the human body, it is absorbed in the intestine, circulated in blood, metabolized in the liver, and then excreted in urine or feces [5,6]. Metabolism of PZQ in the liver is most critical for cure of clonorchiasis because the cure mainly depends upon concentration of hydroxy-PZQ in bile [6]. Since PZQ concentration in blood is widely variable by individuals, its concentration in bile must be more variable due to the blood-bile barrier. That may be supposed the major reason of incomplete cure of clonorchiasis by single PZQ medication.
CYP3A5, which determines activity of hydroxylation of PZQ, is known variable by ethnics. SNP at intron 3 (g.6986A>G; CYP3A5*3) was found 88% among Caucasians but 35% in Africans [7]. Roy et al. [8] recorded SNPs of CYP3A5 in 92.9%, 77.6%, 70.0%, 27.0-50.0%, and 75% of Caucasians, Zimbabweans, Europeans, African Americans, and Asians, respectively. The frequency of total SNPs of CYP3A5 at 7 locations was 17.9% in the 346 Chinese subjects in this study. This rate was lower than those of previous reports [12]. The difference of SNPs is comparable only when same location is observed. There is one record of SNPs at same locations in Caucasians [13]. The SNP frequency was 7.1% and 7.0% in locations of g.-20619T>G and g.-6177A>G among Caucasians while the present study observed rates of 1.7% and 3.5%. The SNP frequency was much lower in the present study than that of Caucasians in several other locations.
The low SNP frequency of CYP3A5 is possibly contributing to high cure rates of PZQ therapy in Asians. The present cure rate was 90.5% of liver fluke disease in Chinese and higher than 60-80% of blood fluke infection in Caucasians [6]. The difference is greatly meaningful because infection of liver flukes is more difficult to cure than that of blood flukes because of the blood-bile barrier [5].
The SNP frequencies of g.-20555G>A and g.27526C>T were 15.2% and 9.1% in the uncured group while those of cured group were 3.8% and 1.0%. The difference was big enough to explain the outcome of PZQ therapy. In other words, it is inferred that individuals with the SNPs of the 2 locations of CYP3A5 may have less concentration of hydroxy-PZQ in their bile. Although it is almost impossible to monitor concentration of hydroxy-PZQ in human bile, this inference is highly plausible. The location of g.27526C>T SNP was in the intron 11, which was not translated. It is still uncertain to explain how untranslated SNPs may be involved in influencing enzyme activities. However, it is presumed to influence stability of the gene during translation. This should be a topic of further studies.
One more correlated significant factor was EPGs of the subjects. The subjects with more EPGs were less cured. EPG counts roughly estimate infection burden of flukes. Therefore, the higher the burden of the liver fluke, the lower the PZQ cure rates were. This phenomenon was suggested by good egg reduction rate (over 95%) of PZQ in clonorchiasis while its cure rate was near 85% [3,4]. Cure of clonorchiasis means complete elimination of flukes from the target human body. Therefore, cure is less possible in individuals with high EPGs than those with low EPGs. Some flukes in humans with high EPGs may survive from PZQ therapy while most of them are eliminated from the body. Previous reports demonstrated that the uncured subjects were all cured by one more PZQ therapy [3,4]. In the present study also, all of the light burden individuals of EPGs less than 100 were cured by one medication. The worm burden is a definite factor which reduces cure rates in clonorchiasis. In this point of view, EPG count itself is more important than SNPs of CYP3A5 for cure.
The limitation of this study was the small number of uncured subjects. A total of 33 uncured individuals were included in this SNPs analysis. The frequencies of SNPs were low, and thus the numbers for analysis were very small. Another limitation of this study was no information of PZQ concentration in blood or bile. If those data were collected together, the present conclusion might have been more concrete.
In conclusion, both EPGs and SNPs of CYP3A5 are factors which influence cure of clonorchiasis by PZQ. It is suggested to recommend 2-day medication for clonorchiasis of individuals with higher EPGs than 1,000 for better cure because SNP analysis of CYP3A5 is not routinely investigated.
ACKNOWLEDGEMENTS
This study was supported by a grant of the Korea Healthcare Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (A030001).