A Paragonimiasis Patient with Allergic Reaction to Praziquantel and Resistance to Triclabendazole: Successful Treatment after Desensitization to Praziquantel
Article information
Abstract
Paragonimiasis is an infectious disease caused by trematodes of the genus Paragonimus. This trematode can be treated successfully with praziquantel in more than 90% of the cases. Although praziquantel is generally well tolerated, anaphylactic reactions to this drug have been reported in a few cases. We report here a 46-year-old Korean female with paragonimiasis, presumed to be due to Paragonimus westermani, who displayed an allergic reaction to praziquantel and resistance to triclabendazole treatment. The patient was successfully treated with praziquantel following a rapid desensitization procedure. Desensitization to praziquantel could be considered when no alternative drugs are available.
INTRODUCTION
Paragonimiasis is an infectious disease caused by Paragonimus. The infection is also known as the lung fluke or pulmonary distomiasis because this trematode normally infects the lungs of mammals [1]. Human infection by the lung fluke Paragonimus westermani is endemic in East Asian and Southeast Asian countries [2-4]. Regardless of the species responsible for the infection, praziquantel is the standard treatment drug and it has a high cure rate [5]. Although praziquantel is regarded as safe, it can induce transient adverse reactions, such as abdominal pain, headache, dizziness, and diarrhea, although allergic reactions to praziquantel are very rare [6-8].
We report a case of complicated paragonimiasis in a Korean female who had a severe allergic reaction to praziquantel. Two courses of triclabendazole treatment failed to cure the infection. The patient was successfully treated with praziquantel following a rapid desensitization procedure.
CASE REPORT
The patient was a previously healthy 46-year-old female who had not traveled to any tropical area during the past 10 years. The patient was first admitted to the Department of Thoracic Surgery, Gachon University Gil Medical Center in mid-August, 2008 with a chief complaint of a 7-day history of fatigue, coughing, and dyspnea on exertion. The patient had no fever or chest pain. The body temperature was normal, respiratory rate was 20 breaths/min, blood pressure was 110/80 mmHg, and pulse rate was 85 beats/min. The right side lung sounds were decreased. The patient's total white blood cell (WBC) count was 10.24×103 cells/mm3 with 24.6% eosinophils, and the erythrocyte sedmentation rate was 8 mm/hr. A chest radiograph showed a marked increase of pleural effusion in the right lung. There were no atypical cells in the pleural fluid, but many degenerated leukocytes were observed. There was no evidence of malignancy. Chest CT of the thorax, which was taken after tube thoracotomy, revealed extensive subcutaneous emphysema in the upper trunk. Examination of stool and sputum samples for parasites and eggs was not performed at this time, and the symptom etiology remained unclarified. Seven days after the tube thorcotomy, the symptoms and roentgenographic abnormalities disappeared, and the patient was discharged.
On October, 2008, she visited our hospital again because of generalized edema, itching, and migrating subcutaneous nodules on the right flank area. The patient complained that these symptoms had persisted for about the previous 1 month. A chest roentgenogram was normal. The ultrasonogram for the right flank showed multiple intramuscular cystic masses that measured up to 1 cm. Although the peripheral blood eosinophil count was elevated (WBC 13.2×103 cells/mm3, eosinophils 27%), the serum total IgE value (54.8 IU/mL) was in the normal range. Concerning dietary history, the patient had ingested incompletely cooked fresh water crab meat 18 months ago, which had been captured from the Sumjin River in South Korea. The serum was tested by microplate ELISA to establish a diagnosis of paragonimiasis [9]. The serum generated a strong positive reaction against Paragonimus westermani, with an optical density (OD) at 405 nm of 0.82 (cut-off OD: 0.25). Praziquantel was prescribed. Twenty min after taking the first dose of praziquantel (1,200 mg/2 tablets, Distocide®; Shinpoong Pharmaceutical Co., Seoul, Korea), the patient experienced a sudden onset of itching sensation, febrile sensation, wheal, and erythematous eruptions on the whole body, and painful swelling on the upper lip and both eyelids. Due of the seriousness of the symptoms, the patient was transferred to the emergency department, where a diagnosis of an allergic or hypersensitivity reaction induced by praziquantel was made. The patient received an injection of 3 mg of piprinhydrinate followed by oral administration of 200 mg piprinhydrinate and 200 mg cimetidine. One hour after receiving this medication, the itching sensation and skin eruption subsided. The patient denied any history of previous exposure to praziquantel. Seven days after this allergic reaction, the patient was given a 10 mg/kg single dose of triclabendazole [10]. The itching sensation gradually disappeared over a period of 1 month after the medication. However, the blood eosinophilia and intramuscular cystic masses in the right flank did not disappear over a period of 4 months after the medication.
On March, 2009 (6 months after the first visit), the patient was readmitted with recurrence of chest pain, coughing, and dyspnea on exertion. A chest radiograph demonstrated a large right-sided pneumothorax with new fluid collection. A tube thoracotomy was performed and this resolved the pneumothorax. The peripheral blood eosinophil count (WBC 5.03×103 cells/mm3, eosinophils 33.1%) and the serum IgE value (652 IU/ml) were elevated. Eosinophilia was also observed in the pleural effusion. P. westermani-specific IgG antibody reaction was strong in the serum (OD: 0.80) and the pleural effusion (OD: 0.70). The patient was treated again with triclabendazole (10 mg/kg once daily for 3 days) together with closed thoracotomy [11]. The patient was discharged on postoperative day 7, and the chest radiograph at that time showed complete re-expansion of the lung with no fluid.
On July, the patient revisited our department because of hemoptysis and chest discomfort. A roentgenogram and CT conducted at that time showed an approximately 3.2×2.8 cm heterogeneously enhanced mass-like consolidation in the anterior segment of the right upper lobe, which was attached to the mediastinal pleura (Fig. 1). The patient underwent a percutaneous needle biopsy of this lung lesion. The histologic findings showed abundant inflammatory cells, including many eosinophils, some granulomas, and necrotic cellular debris (Fig. 2). Parasitological examination of the stool and sputum showed negative results. Triclabendazole-resistant paragonimiasis was strongly suspected on the basis of the persistent eosinophilia (WBC 6.05×103 cells/mm3, eosinophils 27%), radiological findings of the lung (Fig. 1), and histologic findings (Fig. 2). The P. westermani-specific IgG titer (OD: 0.77) was not decreased compared with that noted in October, 2008 (Fig. 3).
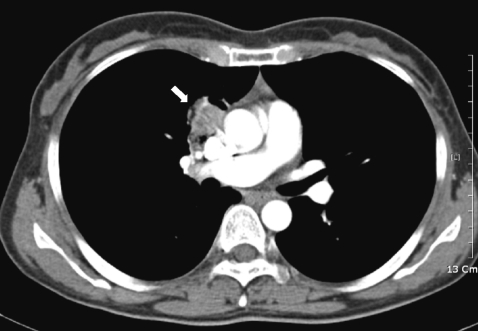
CT scan of the chest of a 46-year-old female diagnosed with P. westermani showed an approximately 3.2×2.8 cm sized heterogeneously enhanced mass-like consolidation (arrow) in the anterior segment of the right upper lobe, which was attached to the mediastinal pleura.
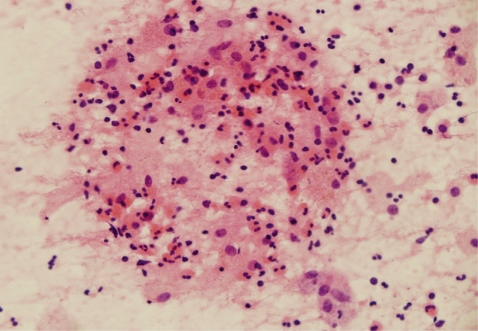
Histologic findings of percutaneous needle biopsy specimens from the lung showed abundant inflammatory cells, including many eosinophils, some granulomas, and necrotic cellular debris. H-E stain, ×400.
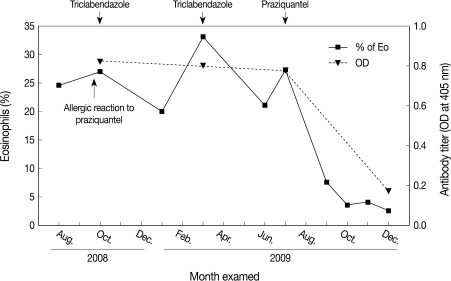
The changes of the percentage of eosinophils and anti-P. westermani IgG antibody titer over time in a 46-year-old female who was diagnosed with P. westermani and received treatments with triclabendazole and praziquantel. The anti-P. westermani IgG titers as measured by micro-ELISA are expressed as the optical density at 405 nm.
On the skin prick and intradermal tests with praziquantel, the patient revealed immediate hypersensitivity to the drug. We decided to try an oral desensitization to praziquantel according to the modified desensitization protocol derived from White et al. [12], with increasing doses (30, 60, 100, 150, 300, 600, and 1,200 mg) administered 90 min apart [12]. Thirty min after the 600 mg dose, she experienced an itching sensation, fever (38.5℃), wheal, and erythematous eruptions on the trunk, and swelling on the upper lip and both eyelids. She received an intravenous injection of 250 mg of hydrocortisone (Solucortef®), 3 mg of piprinhydrinate (Plakon®) and 50 mg of ranitidine. Two hours after receiving this medication, the allergic symptoms and signs subsided. A therapeutic dose of praziquantel (75 mg/kg/day) was then given for 3 days without any symptoms of adverse reactions. The blood eosinophilia gradually normalized over a period of 2 months after the praziquantel treatment.
The chest lesion and cystic mass-like lesions in the flank completely disappeared 4 months after the treatment. The patient remained well for the following 6 months.
DISCUSSION
Human infection with P. westermani is acquired by ingesting fresh water crabs or crayfish that contain the metacercariae. Once ingested, the worms migrate through the intestinal wall to the peritoneal cavity, and then they cross the diaphragm and enter the pleural space and the lung parenchyma. The lung is the primary target of infection, although extrapulmonary infections are also not uncommon [1,13]. The diagnosis is made by the identification of parasites or eggs in tissues, sputum, stool, or pleural fluid. Antibody test by ELISA is a valuable alternative for diagnosing paragonimiasis [14,15]. Regardless of the species that is responsible for paragonimiasis, the standard treatment is chemotherapy with praziquantel (25 mg/kg twice a day, for 2 days) and this is effective in more than 90% of cases. Triclabendazole (10-12.5 mg/kg/time), once or twice a day is recommended as an alternative drug of choice [10]. It is equally effective in deworming Paragonimus and it is better tolerated clinically than praziquantel [10]. The response to chemotherapy with these drugs can be followed by a downward trend of the peripheral eosinophilia and the antibody titer to Paragonimus, the cessation of passing eggs in the stool, and improvement of radiological abnormalities [15,16].
The present case demonstrated complicated clinical features of paragonimiasis. The route of infection was suspected to be the ingestion of raw or incompletely cooked freshwater crab meat during a trip around the Sumjin River in South Korea 18 months previously. At first, the patient was admitted due to pleural effusion. The results of pleural fluid analysis showed only degenerated leukocytes, but the patient had peripheral eosinophilia. The early phase of paragonimiasis involves larval migration within the peritoneal cavity, and penetration into the pleural cavity. In this phase, clinical symptoms are abdominal pain, pleuritic chest pain, pneumothorax, or pleural effusions. Leukocytosis and very prominent blood eosinophilia occur during this time. At that time, the patient's clinical symptoms and marked eosinophilia originally suggested an early phase of paragonimiasis, but diagnostic evaluation for parasitic infestation was not performed. Anti-parasitic treatment was delayed until 9 weeks because the patient underwent ELISA test at the second admission. The delay from the time of the first admission to anti-parasitic drug administration may have resulted in the patient's complicated clinical course of P. westermani infection. At the second admission, the patient complained of a subcutaneous nodule on the right flank area. This lesion was an intramuscular cystic mass that was thought to be an extrapulmonary manifestation of paragonimiasis. Unfortunately, the patient had an allergic reaction to paraziquantel and failed to clear the infection with triclabendazole treatments. During these periods, the patient showed hydropneumothorax and consolidation in the lung. When mature flukes inhabit the lungs during the late phase of paragonimiasis, patients display recurrent hemoptysis and radiologic findings of lungs, such as cystic cavities, parenchymal mass, or pleural thickening. Our patient showed pleural effusion, intramuscular mass, hydropneumothorax, consolidative lesion in the lung, and persistent peripheral blood eosinophilia. We concluded that these all manifestations are caused by paragonimiasis.
A search of the published literature showed that 4 cases of allergic reaction have been attributed to praziquantel treatment [6-8,17]. One report described a 10-year-old male with praziquantel hypersensitivity. The patient was diagnosed as cysticercosis and treated with praziquantel. The patient developed severe urticaria during praziquantel administration. The authors tried desensitization of praziquantel with corticosteroids [8]. Another report described an 11-year-old male with schistosomiasis, who displayed fever, pruritus, and urticaric rash after praziquantel administration. This patient recovered after cessation of praziquantel treatment [17]. A third report described a male patient with clonorchiasis. The patient developed an anaphylactic reaction after praziquantel treatment and was treated with albendazole instead of praziquantel [6]. The anthelmintic efficacy of praziquantel is due to the drug-related exposure of antigens on the tegument of mature and developing parasites [18]. However, it has been suggested that this immunologic reaction to surface antigen from dead worms or eggs may induce host anaphylactic reaction caused by praziquantel [19].
In the present patient, the allergic reaction to praziquantel was demonstrated by skin testing, and the symptoms were reproduced during the desensitization procedure. Although the patient denied previous praziquantel administration, it is possible that a previous exposure to the drug had occurred, producing sensitization to the drug.
To the best of our best knowledge, this is the first successful desensitization treatment with praziquantel that has been described in the literature. Desensitization for drug allergy is the induction of temporary clinical unresponsiveness to drug antigens [20]. Although the exact mechanism of this desensitization is unknown, this can be achieved by administering gradually increasing doses of the allergenic drug at fixed time intervals, and this desensitization allows use of a therapeutic dosage and avoidance of the previous severe drug allergies [12]. One case was previously treated using praziquantel desensitization [8]. However, it is unclear if true desensitization was ever achieved because that patient was concomitantly administrated with corticosteroids during desensitization [8].
In our case of paragonimiasis, the complicated clinical manifestations may have been caused by the delayed diagnosis, the failure of triclabendazole treatment, and the allergic reaction to praziquantel. A desensitization procedure to praziquantel should be considered when no alternative drugs are available.
ACKNOWLEDGEMENTS
The authors thank Dr. Seung-Yull Cho (Gachon University, Incheon, Korea) for critical review of the manuscript, Dr. Sung-Tae Hong (Seoul Nation University, Seoul, Korea) for helpful discussions and Shinpoong Pharmaceutical Company (Seoul) for provision of active pharmaceutical ingredients of praziquantel (Distocide) used in skin test.