Two Cases of Primary Splenic Hydatid Cyst in Greece
Article information
Abstract
Cystic disease of the spleen is an uncommon entity in general population. Most cases result from parasitic infection by Echinococcus granulosus, a form called splenic hydatid disease (SHD), with a reported frequency of 0.5-6.0% within abdominal hydatidosis. On the contrary, an isolated splenic involvement of hydatid disease is very uncommon even in endemic regions. Two cases of primary SHD managed with open and laparoscopic radical surgery in our department are reported herein. Primary SHD is a rare entity with non-specific symptoms underlying clinical suspicion by the physician for prompt diagnosis. Surgical treatment is the mainstay therapy, while laparoscopic approach when feasible is safe, offering the advantages of laparoscopic surgery.
INTRODUCTION
Cystic disease of the spleen is an uncommon entity with an incidence of 0.07% in general population [1]. It is classified as either primary or secondary according to the presence or absence of an epithelial lining of the lumen [2].
The primary cysts are subdivided into parasitic and non-parasitic. Most cases of parasitic cysts result from infection by E. granulosus, a condition called splenic hydatid disease (SHD). SHD constitute up to 6% of cases of abdominal hydatid disease, since the spleen is the third most common affected organ by E. granulosus following the liver and the lungs [3,4]. Humans are the intermediate host and the disease is endemic in the Mediterranean area, New Zealand, Australia, and South America. According to a recent report, human hydatidosis in Greece declines from an annual incidence of 14.8 per 100,000 inhabitants during 1967-1971 to 0.3 in 2008 [5].
Cases of isolated splenic involvement of hydatid disease (HD) are very infrequent even in endemic regions. Other sites such as the heart, pancreas, and muscles are very rarely involved. The development of HD in the spleen is uncommon due to the biological cycle of Echinococcus. A minor percentage (10-20%) of the hexacanth embryos that escape the double liver-pulmonary circulation (first and second Lemman's filters) may spread to any organ or apparatus, including the splenic parenchyma, although other forms of propagation have been hypothesized. Splenic echinococcosis may also arise by retrograde spread from the liver to the spleen via the hepatic portal and splenic veins in portal hypertension [4].
Splenic hydatic cysts can be complicated by secondary infections and fistulization to adjacent organs, even above the diaphragm. Moreover, a systemic anaphylactic reaction which is a life-threatening complication can occur after rupture of the hydatid cyst in the peritoneal cavity [6]. Radical surgery is the mainstay of treatment and chemotherapy is added using anthelmintic regimens to avoid recurrence. In this study, 2 cases of isolated SHD managed with open and laparoscopic splenectomy are presented.
CASES DESCRIPTION
Case 1
A 44-year-old male presented in our department with a 4 week history of a non-specific abdominal pain. Upon his admission, physical examination was unremarkable and routine laboratory tests were within normal limits. Abdominal ultrasound showed enlarged spleen. Abdominal CT followed, which revealed a splenic calcified hydatid cyst, without involvement of any other abdominal organs (Fig. 1A). Immunoblot assay for Echinococcus was positive. A laparoscopic splenectomy, using a 4 port technique with the patient in the right lateral position was performed. The specimen was removed using a retrieval bag through a 7 cm left subcostal incision (Fig. 1B, C).
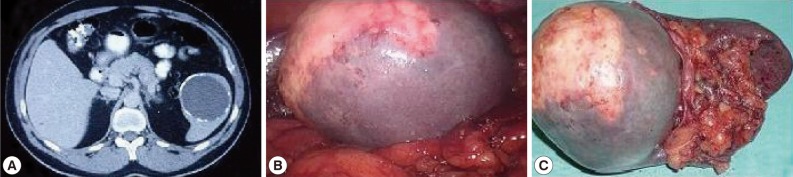
Abdominal CT scan showing the calcified splenic cyst (A), intraoperative view of laparoscopic splenectomy (B), and splenectomy specimen (C).
The patient had an uneventful postoperative course and he was discharged home on the 3rd postoperative day. Albendazole therapy 10 mg/kg/day was started postoperatively and continued for 3 months. One year later the patient remains symptom free and no long-term complications were observed during the follow-up.
Case 2
A 71-year-old female was admitted to our department complaining of early satiety after meals and abdominal discomfort. Physical examination showed a palpable mass or splenic enlargement in the left hypochondrium. Routine laboratory studies were normal. An abdominal ultrasound revealed a calcified splenic mass. Imaging investigation with abdominal CT scan showed a circumferentially calcified spleen (Fig. 2A). Serologic tests for E. granulosus by immunoblot assay were positive. The patient underwent a formal open splenectomy with uneventful postoperative course, and she was discharged home on the 5th postoperative day (Fig. 2B, C). Histopathological results revealed a 9.5 cm cystic cavitary lesion with thick calcified wall, being consistent with hydatid cyst. Chemotherapy with albendazole was used postoperatively, but it was early discontinued due to side effects of abdominal pain and headache, which ended after therapy withdrawal. Patient remains symptom free 18 months after splenectomy.
DISCUSSION
Greece and Eastern Europe are considered endemic regions for the E. granulosus complex [5]. At least 7 of 9 E. granulosus genotypes are infective to humans, 4 of which exist in Europe. Globally, most human cases of cystic echinococcosis are caused by the sheep strain G1 of E. granulosus which predominantly has a dog-sheep cycle [7]. Despite the decrease in rates of infection, Greece stands among the top countries in Europe for hydatid disease prevalence and is considered as a public health issue yet.
Spleen involvement of hydatic disease has no specific clinical manifestations, and the diagnosis is usually established incidentally during investigation of non-specific symptoms. SHD usually co-exists with liver hydatidosis, but rarely as in our cases is the spleen solely affected.
The commonest presentation is discomfort and pain in the left upper quadrant of the abdomen. Early satiety, renal arterial compression with systemic hypertension, spontaneous cutaneous fistulization, or segmental portal hypertension has also been reported as patient's symptoms [7]. This rare presentation can be complicated by secondary infection and fistulization to adjacent organs, even above the diaphragm.
Confirmation of the diagnosis depends on imaging modalities, mostly abdominal ultrasonography and computed tomography (CT scan). Calcification of the cyst wall, presence of daughter cysts, cystic membranes, septa or hydatid sand are imaging findings consistent with SHD. In most cases serologic tests and imaging characteristics in combination indicate the correct diagnosis [8,9]. Hydatid cyst fluid lipoprotein antigen B (AgB) from E. granulosus is considered to be the most specific native or recombinant antigen for immunodiagnosis. The assay for human cysticercosis is based on a family of glycoproteins with molecular weight of 8 kDa with 98% sensitivity and 100% specificity [10].
Treatment is mainly surgical and options depend on individual patient and surgeon's expertise as long as total splenectomy, partial splenectomy, cyst enucleation and unroofing with omentoplasty have all been reported [11]. Percutaneous drainage of the splenic hydatid cyst with injection and consecutive reaspiration of a scolecidal agent (PAIR technique) has been proposed as an alternative, non-surgical therapy for patients at high anesthetic risk or who do not agree to surgery [4]. In addition, splenic salvage is being increasingly advocated to prevent complications associated with splenectomy, mainly an overwhelming infection [2].
A point that has to be emphasized here is whether laparoscopic approach is equally safe to open surgery or not. Scepticism about laparoscopic splenectomy for SHD existed due to concern of cyst rupture and spillage of the parasite with subsequent anaphylactic reaction or recurrent peritoneal hydatidosis. Those concerns have not been proved in reported laparoscopic series, and thus laparoscopic splenectomy for SHD is the mainstay of treatment of this benign disease [12]. Complete splenectomy can be performed safely by laparoscopy (e.g., with hand-assistance), even for huge splenic cysts and offers all the benefits of minimally invasive procedures. However, in our second case, an open approach was preferred because of extensive probable adhesions of the calcified splenic cyst and close relation to the kidney indicated by the preoperative CT-scan.
The role of preoperative administration of anthelmintic therapy in preventing recurrence of the disease is still controversial. Preoperative administration of anthelmintic drugs softens the cysts, reduces intracystic pressure facilitating their removal, and postoperative use reduces the rate of recurrence [13]. According to the WHO guidelines, preoperative administration should begin between 1 month and 4 days before surgery for albendazole and 3 months before surgery for mebendazole. The use of anthelmintic agents may discontinue early, as in our second case, because of side effects. Rash, itching, and facial swellings are signs of allergic reactions which are the main reason of suspending therapy. In addition, persistent dizziness or headache, weakness as well as abdominal cramps, are other common side effects which were presented in our patient [14]. Besides, in the treatment of splenic hydatidosis, postoperative anthelmintic therapy may or may not be used in case of splenectomy which is performed without any spillage of the cyst contents [15].
Isolated SHD can be presented with non-specific symptoms underlying clinical suspicion by the physician for prompt diagnosis which is usually late. Treatment is mainly surgical by laparoscopy, while organ-preserving procedures are achievable only in cases with early diagnosis.
