Effect of Temperature on Embryonation of Ascaris suum Eggs in an Environmental Chamber
Article information
Abstract
The influence of temperature on the development and embryonation of Ascaris suum eggs was studied using coarse sand medium in an environmental chamber with 50% humidity. The time required for development and embryonation of eggs was examined under 3 different temperature conditions, 5℃, 25℃, and 35℃. A. suum eggs did not develop over 1 month at the temperature of 5℃. However, other temperature conditions, 25℃ and 35℃, induced egg development to the 8-cell-stage at days 5-6 after incubation. All eggs examined developed to the 8-cell stage at day 6 after incubation in the sand medium at 25℃. The higher temperature, 35℃, slightly accelerated the A. suum egg development compared to 25℃, and the development to the 8-cell stage occurred within day 5 after incubation. The formation of larvae in A. suum eggs at temperatures of 35℃ and 25℃ appeared at days 17 and 19 after incubation, respectively. These findings show that 35℃ condition shortens the time for the development of A. suum eggs to the 8-cell-stage in comparison to 25℃, and suggest the possibility of accelerated transmission of this parasite, resulting from global warming and ecosystem changes.
Ascaris lumbricoides, a soil-transmitted nematode, can cause human infections through ingestion of food contaminated with embryonated eggs [1]. Human infection is prevalent in countries having warm and moist soil in tropical and subtropical countries. This parasite is an important public health burden because most of the people infected with this parasite are children in developing countries who often are malnourished [1]. For the parasite management, we need to know factors affecting the development of parasite eggs in soil, such as temperature and humidity. Recent studies on soil-transmitted nematodes, especially the genus Ascaris, included those on A. suum in pigs as a model for A. lumbricoides infection of humans due to several reasons [2]. A. lumbricoides and A. suum are closely related ascarid species and both species constitute natural host-parasite relationships with similar life cycles. In addition, an in vitro test for embryonation of eggs showed that A. suum eggs could be used as a model for A. lumbricoides [3,4].
Global climate change is resulting in changes in sea water and soil, and furthermore, will affect soil- and water-borne parasitic diseases [5,6]. The effects of climate change on the ecosystem of parasites have been of particular concern. In particular, the embryogenesis of helminth eggs depending on the ambient temperature can be measured to predict prevalence of soil-transmitted helminths [7]. A. suum is a soil-transmitted nematode which can infect pigs and cattle, and it is known that the egg development and survival are affected by temperature [7-11].
In the Republic of Korea (=Korea), the infection rate of A. suum in pigs was reported to be about 17.6% by fecal examination in rural areas [12]. A. suum is an important zoonotic parasite, and genetic studies showed that A. suum may infect humans also [12-14]. The infection rate of 17.6% in pigs is enough to raise concern for environmental hygiene. Recently, A. suum eggs were studied for the effect of temperature on embryostasis of eggs in kimchi extract to investigate larval development during storage of kimchi [15].
In consideration of the close relationship of global warming and soil-transmitted helminth infections [5], studies on the effects of temperature for the development and embryonation of helminth eggs have been performed. A study using A. suum eggs reported that all embryos observed were in the second stage larvae (L2) on day 18 after incubation at 28℃ [4]. In the same study, the appearance of early-morular stage was at days 3 to 7 after incubation, and a pre-larval stage appeared at days 9 to 13 [4]. Given that morphological characteristics detected by microscopy are from the 1-cell to 8-cell-stages (further to morular stage), the tracking of egg development could be completed within 7 days.
The present study was performed to investigate the degree of development and embryonation of A. suum eggs in low, medium, and high temperature conditions. For this purpose, we used an environmental chamber supplying oxygen to eggs and coarse sand medium mimicking soil. Eggs in the chamber soil were microscopically examined daily.
To collect A. suum eggs, the adult worms were harvested from the intestines of naturally infected pigs at a slaughterhouse in Pocheon-si, Gyeonggi-do, Korea. Worms were kept in PBS for 24 hr at 25℃ to allow spawning. A. suum eggs in PBS were collected for 5 days by replacing fresh PBS every day. Eggs collected were washed with PBS and stored at 4℃ for further use. To provide an experimental environment similar to soil, we designed a conditioned soil using coarse sand. The sand purchased from an aquarium was washed thoroughly with water to remove light debris and then passed through a 1-mm sieve. The sand was autoclaved for 1 hr to kill all pathogens and divided into 30 g in a Petri-dish. Finally, eggs were scattered on coarse sand medium to become 2,000 eggs per dish and the dish was shaken several times to mix well both sand and eggs. The environmental chamber, TH-ME-065 (JEIO Co, Incheon, Korea), was continually maintained in each temperature condition (5℃, 25℃, and 35℃) until embryonation was observed. Humidity was controlled at 50% in all temperature conditions. The developmental stages of the eggs were observed by an optical light microscope, CHS-213E (Olympus, Tokyo, Japan). To count the developed eggs, 50 eggs were recovered from the Petri-dish and counted for 1-cell- to 8-cell-stage eggs.
The results showed that the eggs did not develop over 1 month period at 5℃ and continued to be at the 1-cell stage (Fig. 1A). However, when the culture was continued for 3 months, embryonic development appeared slowly (data not shown). Under 25℃, the eggs developed and appeared to be at 2-cell- to 8-cell stages during day 2 to day 6 after incubation (Fig. 1B). On day 2, 13 of 50 eggs were converted from 1-cell- to 2-cell-stage (26%) and 1 egg developed to the 4-cell stage (2%). The other 36 eggs remained at the 1-cell-stage (72%). On day 3, 26 eggs developed to the 2-cell-stage (52%) and 16 eggs to the 4-cell-stage (32%). Likewise, 24 eggs developed to the 4-cell-stage (48%) on day 4, and 20 eggs to the 8-cell-stage (Fig. 2B). Only 1 egg remained to be in the 1-cell-stage (Fig. 2B). On day 5, 38 eggs developed to the 8-cell-stage (78%) which soon progressed to the morular stage, and 10 eggs and 2 eggs were at the 4-cell- and 2-cell-stages, respectively. On day 6, all eggs developed to the 8-cell- or morular stage. Under 25℃, embryonated eggs possessing a larva were observed on day 19 after incubation (data not shown).
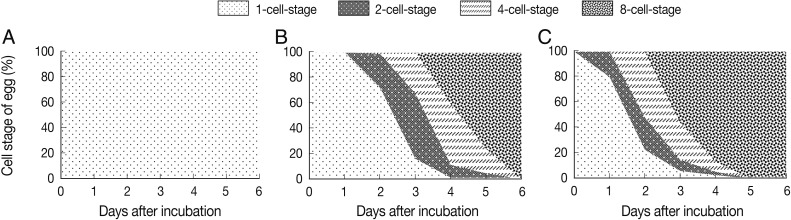
The effect of temperature on the development of Ascaris suum eggs in an environmental chamber with 50% humidity. The degree of A. suum egg development at the temperature of 5℃ (A), 25℃ (B), and 35℃ (C). A. suum eggs were scattered on coarse sand medium and incubated at each temperature condition. To count developed eggs, 50 eggs were randomly collected every day and observed by microscopy. Data shows the percentage of each cell-stage in each temperature condition.
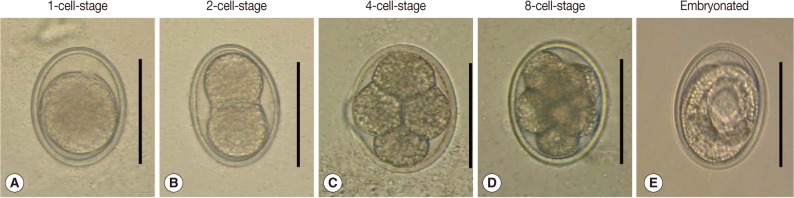
Developmental stages of A. suum eggs incubated in the environmental chamber. (A) 1-cell-stage, (B) 2-cell-stage, (C) 4-cell-stage, (D) 8-cell-stage (early-morular stage), and (E) an embryonated egg possessing a larva. Scale bar=50 µm.
At a higher temperature condition, 35℃, eggs developed slightly faster than at 25℃ (Fig. 1C). Some of the eggs rapidly developed to the 2-cell-stage at 35℃ from day 1 after incubation compared to 0% that did at 25℃ (Fig. 1B, C). At day 1, 20% of the eggs were at the 2-cell-stage. From day 2, some eggs started to develop into the 4-cell-stage, and on day 3, 28 eggs reached to the 8-cell-stage (56%) (Fig. 1C). This tendency of egg development represented that 35℃ compared to 25℃ temperature condition accelerated the process of egg embryonation. At day 5, all eggs reached to the 8-cell- and morular stages. Embryonated eggs possessing a larva were observed from day 17 after incubation (data not shown). The morphology of all developmental stages of the eggs is shown in Fig. 2.
A recent paper reported morphological changes of A. suum eggs during incubation in vitro with 0.1 N H2SO4 at 28℃ [4]. The study described 12 different developmental stages of A. suum eggs, from the 1-cell-stage to L2 [4]. It was shown that, on day 14 of incubation, 90% of the eggs developed to the first stage larva (L1), and by day 21, 100% developed to L2 [4]. However, the study did not reflect a natural environmental condition because the eggs were placed in a centrifuge tube with 5 ml of 0.1 N H2SO4. In the present study, we tried to design a natural environment to observe the survival and development of A. suum eggs using an environmental chamber and the coarse sand medium. In the chamber, the eggs were in contact with atmospheric oxygen, the specified humidity, and the determined temperature depending on the experimental condition. Our results showed that the early development of A. suum eggs during the first 6 days after incubation was affected by temperature. Because A. suum is a soil-transmitted nematode, the conditioned coarse sand medium used in this study may have played a role for soil in nature. Previous studies on the early development of A. suum eggs usually used saline or other liquid, such as 0.1 N H2SO4, for in vitro incubation [4,16]. These humidity conditions did not reflect natural soil humidity. Meanwhile, the eggs of Toxocara canis, another soil-transmitted nematode, developed well in the presence of 50% moisture [17]. A high humidity of 50% accelerated the egg development to infective stage eggs (66.8%) in comparison to a dry condition of 3% humidity [17]. Accordingly, we adjusted the relative humidity to be 50% inside the chamber as the proper humidity.
Results in the present study showed some differences in the egg development between 25℃ and 35℃. At the temperature of 35℃, all eggs developed to the 8-cell-stage by day 5. On the other hand, at 25℃, 24% of the eggs remained at the 2-cell- to 4-cell-stages at day 5 after incubation, although 76% converted to the 8-cell-stage. Meanwhile, in another study, various culture conditions were attempted to compare the development of A. suum eggs by different temperatures [8-10,16]. The results showed that 31±1℃ induced the maximum rate of egg development in the culture medium of 0.1 M H2SO4 using a 50-ml conical flask, but the lower temperature ranging 16-25℃ also showed development of eggs to a certain degree [16]. In the present study, development of eggs did not occur at the temperature of 5℃ over a 1 month experimental period. However, they were found to develop to a larval stage when observed until 3 months (data not shown). This result was similar to the development of T. canis eggs at 4℃ incubation [17]. These results mean that a low temperature delay the development of soil-transmitted nematode eggs, but cannot completely eliminate their potential of development.
Recent global warming resulted in climate changes in subtropical areas with increases in temperature and humidity. Korea is now changing into a subtropical climate zone [18]. A change of 1-2℃ in the annual mean or winter mean temperature over the region is significant enough to cause an impact in the environment, particularly the growth rate of plants, bacteria, viruses, and so on [18]. Previous data showed that embryonation of A. suum and Trichuris suis eggs took place only during the months with temperature ranging from 10℃ to 30℃ [7]. Therefore, propagation of soil-transmitted parasites can be increased by warming of the soil as shown by our results. Since Korea was an endemic area for soil-transmitted helminthiases for a long time [19], the change of Korea to a subtropical region may exert a significant impact on re-transmission of these parasites by accelerating the egg embryonation in the environmental condition.
In conclusion, our results showed that the embryonation of A. suum eggs was highly limited at the temperature of 5℃. On the other hand, 35℃ temperature condition accelerated the embryonation of eggs more prominently than 25℃. The results suggest that the increase of temperature by global warming may exert to increase in the prevalence of soil-transmitted nematodes through accelerating the embryonation of Ascaris eggs in soil.
ACKNOWLEDGMENTS
This work was supported by a grant (10162KFDA995) from the Korea Food and Drug Administration in 2011.