Imported Intraocular Gnathostomiasis with Subretinal Tracks Confirmed by Western Blot Assay
Article information
Abstract
We report a case of intraocular gnathostomiasis diagnosed by western blot assay in a patient with subretinal tracks. A 15-year-old male patient complained of blurred vision in the right eye, lasting for 2 weeks. Eight months earlier, he had traveled to Vietnam for 1 week and ate raw wild boar meat and lobster. His best-corrected visual acuity was 20/20 in both eyes and anterior chamber examination revealed no abnormalities. Fundus examination showed subretinal tracks in the right eye. Fluorescein angiography and indocyanine green angiography showed linear hyperfluorescence of the subretinal lesion observed on fundus in the right eye. Ultrasound examination revealed no abnormalities. Blood tests indicated mild eosinophilia (7.5%), and there was no abnormality found by systemic examinations. Two years later, the patient visited our department again for ophthalmologic evaluation. Visual acuity remained 20/20 in both eyes and the subretinal tracks in the right eye had not changed since the previous examination. Serologic examination was performed to provide a more accurate diagnosis, and the patient's serum reacted strongly to the Gnathostoma nipponicum antigen by western blot assay, which led to a diagnosis of intraocular gnathostomiasis. This is the first reported case of intraocular gnathostomiasis with subretinal tracks confirmed serologically using western blot in Korea.
INTRODUCTION
Gnathostomiasis is a parasitic zoonosis that rarely occurs in humans [1]. Thirteen species of Gnathostoma have been reported to date, of which 5 species such as Gnathostoma spinigerum, G. nipponicum, G. hispidum, G. doloresi, and G. binucleatum infect humans [2]. Humans become incidental hosts by ingesting raw fish or meat infected by the third-stage larvae and the larvae migrate through the internal organs and skin [3,4]. The mechanisms for clinical manifestations of human gnathostomiasis include mechanical damage of the host tissues caused by migration of the parasitic larvae and host responses to Gnathostoma toxins resembling those of protease, hyaluronidase, acetylcholine, and hemolysin [5]. Skin infection manifested by local pain and migratory swelling in the skin and subcutaneous tissues is the most common form of human gnathostomiasis. Of the forms of visceral gnathostomiasis, lethal meningitis is the commonest, but intraocular infection can rarely occur, even many years after the initial infection [6]. Ocular gnathostomiasis has been reported to involve the eyelid, conjunctiva, cornea, anterior chamber, and vitreous cavity. Only 24 cases of ophthalmic gnathostomiasis have been reported to date; 14 cases in the anterior segment, 6 cases in the posterior segment, and 4 cases in the orbital tissues and eyelid. Among these, subretinal tracks in the posterior segment were reported in 1 case [7].
Identification of the larvae is required for a definite diagnosis of human gnathostomiasis, but it is difficult to find the larvae. Therefore, diagnosis depends largely on clinical manifestations and history of potential ingestion of a host infected with the larvae. Recently, serologic tests, such as western blot assay, became available for diagnosis [8]. Here, we report a case of intraocular gnathostomiasis diagnosed with western blot assay in a patient with subretinal tracks observed on fundus examinations, although no larvae were found.
CASE RECORD
A 15-year-old male patient complained of blurred vision in the right eye, which started 2 weeks earlier. He had traveled to Vietnam 8 months ago and ate raw wild boar meat and lobster during his 1-week stay there. The best-corrected visual acuity was 20/20 in both eyes. He was orthophoric with a full range of ocular movements and the pupil light reflex was normal in both eyes. Slit lamp examination revealed no abnormalities, but fundus examination revealed irregular linear lesions in the entire fundus of the right eye and no abnormalities in the left eye. Ultrasonography and electroretinography revealed no abnormalities. Fluorescein angiography identified a linear hyperfluorescence in the lesion and indocyanine green angiography revealed engorgement of some choroidal vessels and linear hyperfluorescence in the same lesion (Fig. 1).
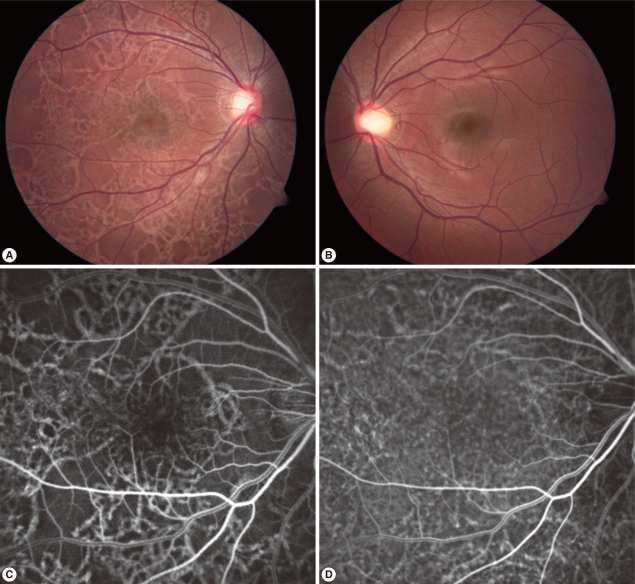
Fundus photographs of both eyes of the patient, fluorescein angiography (FAG), and indocyanine green angiography (ICGA) of the patient's right eye at presentation. (A) Fundus photographs of the right eye showed criss-crossing subretinal migratory and hypopigmented tracks and multiple pigmented lesions in all quadrants at presentation. (B) Fundus photographs of the left eye showed no abnormal finding. (C) Fluorescein angiography of the right eye showed diffuse crisscrossing hyperfluorescence. (D) Indocyanine green angiography of the right eye showed diffuse crisscrossing hyperfluorescence and engorgement of some choroidal vessels.
The patient had normal consciousness and no abnormal neurologic findings. Eosinophils were mildly elevated at 7.5% in blood tests, but routine stool and urine examinations revealed negative for parasite infections. The patient was treated with 400 mg of albendazole (Alzentel®; Shin Poong Pharm. Co., Seoul, Korea) twice daily for 21 days for a parasitic infection which was suspected based on the subretinal lesions in the right eye and the patient's travel history, despite the fact that we did not detect any nematode larvae. We were going to perform a surgical treatment if larvae were found in the fundus at the follow-up examination. For the following 2 months, larvae were still not observed, visual acuity remained at 20/20, and fundus examination showed no significant changes in the lesion.
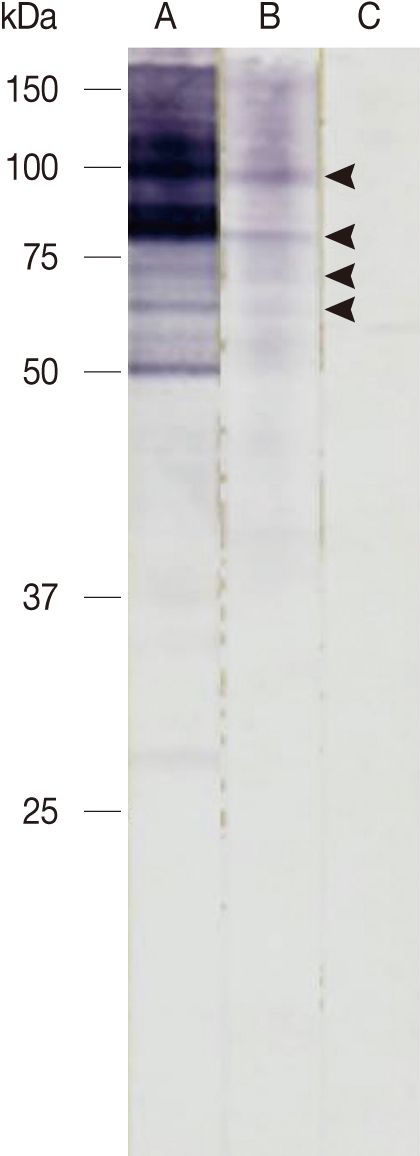
Two years later, he visited our clinic again for an ophthalmic examination. Visual acuity was 20/20 in both eyes, hypopigmented linear lesions were observed across the whole fundus in the right eye on fundus examination, and subretinal tracks were observed on optical coherence tomography (Fig. 2). Because subretinal tracks can be found in several different types of parasitic infections and thus are not diagnostically specific. Based on the history and clinical infection of the patient, we suspected the infection of Gnathostoma spp. and performed western blot analysis. Western blot assay was carried out with the soluble lysate of G. nipponicum. In brief, the soluble lysate of G. nipponicum was separated by 10% SDS-PAGE and transferred to nitrocellulose membrane. The membrane was cut into strips and blocked with PBS supplemented with 0.05% Tween 20 (PBST, pH 7.4) and 3% skim milk for 1 hr at room temperature. The strips were then incubated with a 1:50 or 1:100 diluted positive serum collected from a patient with gnathostomiasis, a serum from this patients, and a serum of normal healthy person for 2 hr [9]. After several washes with PBST, the strips were incubated with 1:1,000 diluted horseradish peroxidase-conjugated anti-human IgG (Sigma, St. Louis, Illinois, USA) and the immunoreactive bands were visualized with 4-chloro-1-naphthol (Sigma). Several immunoreactive bands ranged from 50-100 kDa, which suggested positive antibody responses against the antigens of the parasite, were identified (Fig. 3). Other serologic tests with molecular band for antigens from Angiostrongylus, Paragonimus, Toxocara, and other parasitic origins were performed. Serologic results for other parasites were negative. Collectively, we made a diagnosis of intraocular gnathostomiasis based on the history of ingesting raw wild boar meat and lobster in an endemic area of gnathostomiasis, as well as the mild eosinophilia and positive western blot assay result.
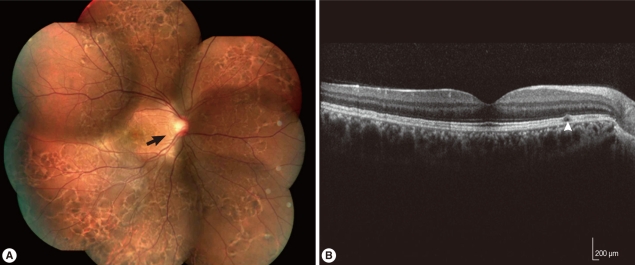
Fundus photograph and optical coherence tomography (OCT) 2 years after the initial presentation (A) Photograph of the right eye showed crisscrossing subretinal migratory and hypopigmented tracks (arrow) and multiple pigmented lesions in all quadrants. (B) Optical coherence tomography of the right eye showed subretinal tracks (arrowhead) between the retinal pigment epithelium and inner segment/outer segment of the photoreceptors.
DISCUSSION
In general, intraocular parasitic infections reported to date include gnathostomiasis, toxoplasmosis, toxocariasis, Acanthamoeba keratitis, cysticercosis, onchocerciasis, and ophthalmomyiasis [10,11]. Symptoms of intraocular gnathostomiasis include lid swelling, iritis, iris atrophy, iris hole, retinal scarring (subretinal tracks), and retinal detachment. In particular, Biswas et al. [7] found that subretinal tracks were multiple yellow linear tracks, which suggested the migratory path of the subretinal parasite, and were seen subretinally in the posterior pole and in the periphery, but no parasite was actually seen.
Toxoplasmosis is the most common cause of posterior uveitis worldwide caused by Toxoplasma gondii. It causes focal necrotizing retinochoroiditis involving retina often associated with a preexistent chorioretinal scar and variable involvement of the vitreous, retinal blood vessel, optic nerve, and anterior segment of the eye [12]. Ocular toxocariasis is a worldwide public health problem caused by larvae of Toxocara canis or Toxocara cati. Clinically, the lesion appears as a whitish mass involving the retina and peripheral vitreous. Other signs include endophthalmitis and posterior pole granuloma [11]. A definite diagnosis of ocular toxocariasis is difficult to establish since the larvae is rarely identified from the lesion. The current gold standard to test for ocular toxocariasis is ELISA, which carries both a high sensitivity and specificity rate of approximately 90% [13]. Park et al. [14,15] reported 6 cases of ocular toxocariasis confirmed by serology in Korea and that positive serology supported the diagnosis if clinically compatible. Acanthamoeba keratitis is a serious corneal infection caused by the genus Acanthamoeba and has been exponentially increasing in recent years correlating to the growing number of contact lens wearers, even though considered a relatively rare disease in comparison with other forms of infectious (fungal or bacterial) keratitis [11,16]. Ocular cysticercosis gets localized at eyelids, subconjunctival tissues, anterior chamber, or in the vitreous cavity. A translucent cyst with characteristic undulating movements by the cyst gives the name "living pearl". On rupture of the cyst, the patient may develop anterior uveitis, vitritis, and rhegmatogenous retinal detachment [11]. Ocular onchocerciasis is prevalent along the river, hence it is called "river blindness". Clinical manifestations include limbal edema, hyperemia, keratitis, bilaterally symmetrical chronic chorioretinitis [11,17]. Ophthalmomyiasis is initiated by parasitic dipterous fly larvae feeding in the host's necrotic or living tissues. Especially, ophthalmomyiasis interna is characterized by pigmented and atrophic retinal pigment epithelium tracts in a criss-crossing pattern seen in conjunction with hemorrhage and fibrovascular proliferation. However, subretinal larvae are generally found because of 10 to 20 mm sized larvae [10]. Two likely causes of subretinal tracks include intraocular gnathostomiasis and ophthalmomyiasis [7,10]. However, a differential diagnosis between intraocular gnathostomiasis and ophthalmomyiasis is difficult, we highly suspected intraocular gnathostomiasis based on past history and fundus findings.
Gnathostomiasis is found both in endemic and non-endemic areas, which makes it an important disease not only for those who live in the endemic areas but also for those who travel to these areas [4]. Thailand, Vietnam, Myanmar, and Japan are endemic areas and some cases of gnathostomiasis were recently reported in Europe and Africa [3,18,19].
In general, gnathostomiasis causes migratory edema in the skin, but may involve other organs in the body and can even involve the central nervous system or the eye, although such cases are rare [7]. Twenty-four cases of intraocular gnathostomiasis have been reported to date and most were found in the anterior segment. Six cases involved the posterior segment, causing panuveitis or vitreous opacity (Table 1) [5-7,20-37]. One very rare case was reported, in which an extensive and irregular subretinal tracks was found that was not accompanied by chorioretinal hemorrhage [7]. The mode of entry of intraocular parasites causing intraocular gnathostomiasis is not clear, but it is suspected that larvae invade through blood vessels, based on hemorrhagic spots found in the central retinal artery. In another case, the larvae might have directly invaded the subretinal space, similar to the case presented here, leaving extensive chorioretinal scars [7].
In principle, the diagnosis of intraocular gnathostomiasis is made based on the larvae found during follow-up. The larvae is about 2 mm long and 0.3 mm wide, and motile [26]. It is extremely difficult to find larvae, and many cases of intraocular gnathostomiasis are not diagnosed. Diagnosis of gnathostomiasis with no larvae detected depends on the clinical manifestations, past history of ingesting raw or semi-cooked meat or fish in endemic areas or travel sites, intermittent subcutaneous migratory swelling, and eosinophilia. Migratory skin lesions or eosinophilia, however, may not be present in intraocular gnathostomiasis [29]. Western blot assay has recently become a widely used tool for diagnosing gnathostomiasis. Designed to find IgG class antibodies in human sera, western blot assay is a very valuable diagnostic test tool due to its high sensitivity (91.6-92.9%) and specificity (87.8-93.4%), although the sensitivity and specificity may differ slightly between studies, depending on the antigens from the Gnathostoma species [8,18]. Ikadai et al. [38] reported that somatic antigens of G. nipponicum might be especially relevant in the search for a better immunogenic protein for the diagnosis of G. nipponicum infection.
Finding subretinal tracks does not necessarily lead to the conclusive diagnosis of parasitic disease. Subretinal tracks involving choroidal macrovessels were observed on fundus examination in 1 reported case [39]. If subretinal tracks are found, a parasitic disease is suspected, and in our case, choroidal macrovessels and other retinal vascular diseases are excluded based on the fluorescein angiographic and optical coherence tomographic findings. In addition, the travel history of the patient to the endemic area was helpful in leading to the diagnosis of a parasitic disease. Fundus findings of the subretinal tracks, however, were not diagnostically specific and mild eosinophilia in the blood count alone was not sufficient for a definitive diagnosis. We suspected the patient as having gnathostomiasis because he stayed in Vietnam for 1 week, consumed raw wild boar meat and lobster there, and the eye was involved. A serologic test for Gnathostoma antigen was conducted, followed by other serologic tests for Angiostrongylus, Paragonimus, Toxocara, and other parasites, in order to find cross reactivity with gnathostomiasis [4,15]. The serologic tests for other parasites were negative and western blot assay was used to examine the reactivity of the patient's serum to Gnathostoma antigen compared with a positive reference serum for gnathostomiasis. The results revealed 4 reactive bands which were identical in the 2 samples.
In this paper, we report a case of intraocular gnathostomiasis diagnosed based on rare clinical findings of subretinal tracks, past history of ingesting raw meat in an endemic area, mild eosinophilia, and positive western blot result.
ACKNOWLEDGMENTS
None of the authors has conflict of interest with the submission. No financial support was received for this submission.