Mactra veneriformis, an Intertidal Clam, as a New Second Intermediate Host for Acanthoparyphium marilae (Digenea: Echinostomatidae)
Article information
Abstract
Metacercariae of Acanthoparyphium marilae Yamaguti, 1934 (Digenea: Echinostomatidae) were discovered in an intertidal clam, Mactra veneriformis, in a southwestern coastal area of the Republic of Korea. A total of 128 metacercariae were detected from 10 clams examined. They were round, 320 µm in average diameter, with 23 collar spines. They were fed experimentally to chicks, and 10 days later adult flukes were obtained. The adults were morphologically characterized by the head collar with a single row of 23 dorsally uninterrupted spines, without special end group spines, a round ventral sucker, 2 round and tandem testes, and vitellaria extending at lateral fields from the posterior extremity not beyond the middle level of the posterior testis. The most characteristic feature of this species was the limited distribution of vitellaria, which differs from Acanthoparyphium tyosenense Yamaguti, 1939, the metacercariae of which are encysted in the same mollusk species. This is the first report in which the metacercariae of this species were detected, and the intertidal bivalve, M. veneriformis, has been identified as a second intermediate host for A. marilae.
The genus Acanthoparyphium (Digenea: Echinostomatidae) is a group of minute intestinal trematodes which parasitize the small intestine of aquatic birds in marine areas of the Republic of Korea, Japan, USA, the Philippines, Kuwait, India, Puerto Rico, and Australia [1-8]. Brackish water gastropods, bivalves, or oysters play the role of a first or second intermediate host, or both [1-8].
In the Republic of Korea, Chai et al. [7] reported recently that Acanthoparyphium tyosenense Yamaguti, 1939 causes human infections. The complete life history of A. tyosenense has been previously elucidated; several species of brackish water mollusks have been demonstrated to harbor the metacercariae of A. tyosenense [7,9,10]. The adult parasite of A. tyosenense was identified originally in velvet scoters, Melanitta fusca stejnegeri, and common scoters, Melanitta nigra americana, as natural infections in Korea [11]. Its life cycle was suggested also in Japan [10].
Acanthoparyphium marilae Yamaguti, 1934 was originally described in velvet scoters (Marila marila mariloides and M. fusca stejnegeri) in Japan [12], and thereafter in common scoters (M. nigra americana) in Korea [11], and greater scaups (Aythya marila mariloides) and great knots (Calidris tenirostris in China [13]. Although intertidal mollusk species are generally known to harbor the metacercariae of Acanthoparyphium species, the intermediate host for A. marilae remained to be determined. In the present study, Mactra veneriformis, a common intertidal bivalve species in the Republic of Korea, was subjected to a search for Acanthoparyphium metacercariae in order to determine the intermediate host for A. marilae.
The metacercariae of A. marilae, together with those of A. tyosenense and Himasthla alincia, were detected in the bivalve, M. veneriformis, which were purchased in a southwestern costal area, Buangun, Jeollabuk-do, the Republic of Korea (Table 1). The metacercariae were isolated from the muscles and gills of the bivalves with a sharp, pointed pin and a stereomicroscope. After placing them on glass slides, a cover slip was placed on the specimen and gentle pressure was applied. The metacercariae were then identified individually using a light microscope. The metacercariae of A. marilae were discriminated from those of A. tyosenense by their smaller cyst size, and average diameters of 305-330 µm for A. marilae (this study) and 420-460 µm for A. tyosenense [7].

Infection status of echinostomatid metacercariae by body portions of the bivalve, Mactra veneriformis
A total of 128 A. marilae metacercariae were recovered from 10 M. veneriformis, with an infection rate of 100% and an average number of 12.8 metacercariae per clam (Table 1). The metacercariae were coiled in thin-walled, oval to round cysts, 320 µm in average diameter, with branched excretory tubules and granules, and with transparent cyst walls (Fig. 1). Each was equipped with an oral and a ventral sucker, and around the oral sucker were 23 collar spines arranged in a single row, without ventral corner spines.
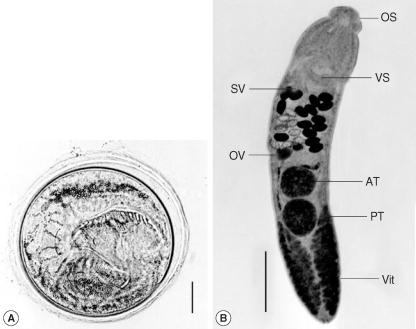
(A) A metacercaria of Acanthoparyphium marilae from an intertidal clam, M. veneriformis, which shows refractile excretory granules and collar spines (Bar: 100 µm). (B) An adult (10-day-old worm) of Acanthoparyphium marilae recovered from an experimentally infected chick (Bar: 500 µm). The body is elongated and the genitalia are fully matured. The vitellaria extend from the posterior end to the middle level of the posterior testis.
OS, oral sucker; SV, seminal vesicle; VS, ventral sucker; OV, ovary; Vit, vitellaria; AT, anterior testis; PT, posterior testis.
A hundred metacercariae were administered orally to 5 experimental chicks (3-day-old, Gallus domesticus), and the chicks were killed on day 10 post-infection (PI). Their small intestines were resected, longitudinally opened in a Petri dish containing saline, and examined for worms using a stereomicroscope. The adult flukes collected were then fixed in 10% neutral formalin under a cover glass pressure and stained with Semichon's acetocarmine. The stained specimens were observed and measured microscopically. All measurements are given in micrometers, unless stated otherwise.
Ten adult flukes (recovery rate, 10%) were recovered from the small intestine of chicks on day 10 PI, and the worms were identified as A. marilae, on the basis of the following morphological features and measurements (n = 10) (Table 2).

Comparative measurements of Acanthoparyphium marilae adults recovered from the chicks with those described by previous workers
Description: Body elongated, tapering anteriorly, and rather elliptical at the posterior end. Head collar armed with 23 spines arranged in a single row, without ventral corner spines. Round acetabulum at the posterior end of the anterior third of the body. Cirrus sac characteristically passing beyond posterior margin of the ventral sucker. Vitellaria distributed along the ceca from the middle level of the posterior testis to the posterior end of the body. Genital pore just in front of the acetabulum. Two testes tandem, and located near the middle portion of anterior part of the posterior half of the body. Oval ovary located a little to the right of the median line between the cirrus pouch and anterior testis. Eggs immature, ovoid to elliptical, with a thin wall and a less prominent operculum.
Acanthoparyphium marilae Yamaguti, 1934 was originally described based on specimens recovered from the small intestines of ducks and knots in Korea and Japan [11,12]. Its intermediate host was previously unknown. In the present study, M. veneriformis, a species of intertidal bivalves, has been determined to harbor the metacercariae of A. marilae; the adult flukes were obtained from an experimental infection to chicks.
The taxonomic identification of the adult specimens from the chicks was not difficult. The worms revealed a prominent head collar with 23 cephalic spines arranged in a single row, without grouped corner spines, a long cirrus sac reaching beyond the posterior margin of the acetabulum, round and entire testes, and vitellaria distribution limited posteriorly to the level of the posterior testis. On the basis of these characteristics, the specimens were assigned to a species of Acanthoparyphium [14], and identified as A. marilae Yamaguti, 1934 [12].
A total of 14 species of Acanthoparyphium Dietz, 1909 are known in the literature [14,15], which include A. phoenicopteri Diez, 1909, A. spinulosum Johnston, 1917 (a synonym: A. spinulosum suzugamo Yamaguti, 1939), A. ochthodromi Tubangui, 1933, A. marilae Yamaguti, 1934, A. squatarolae Yamaguti, 1934, A. charadrii Yamaguti, 1939, A. kurogamo Yamaguti, 1939, A. melanittae Yamaguti, 1939, A tyosenense Yamaguti, 1939, A. parachardrii Velasquez, 1964, A. loborchis Wang, 1977, A. haematopium Ku and Chiu, 1979 and A. macracanthum Rybakov and Lukomskaya, 1988. In the species determination for our specimens, the level of vitellaria extension appeared to be the most salient clue. The vitellaria distribution of our specimens was confined to the middle level of the posterior testis; this feature was unique only to A. marilae, among the 14 species. In addition, testes morphology and the position of the ventral sucker were also useful features.
The natural definitive hosts of Acanthoparyphium are always aquatic birds, and the majority of them are migratory; some of them, like plovers, godwits, knots, and dotterels fly from Siberia to southern Australia [1-5]. Human infections were reported only in 1 species, A. tyosenense [7]. The life histories of A. spinulosum, A. paracharadrii, A. macracanthum, and A. tyosenense have been previously studied [2,5,7,10,13].
The second intermediate hosts of Acanthoparyphium species have been identified as intertidal mollusks, snails, and oysters, namely, Ruditapes philippinarum, M. veneriformis, Solen grandis, and Solen strictus for A. tyosenense [7,10], Certhidea californica, Crassostrea virginica, Pyrazuz australis, and Salinator fragis for A. spinulosum [2], Cerithium ornate for A. paracharadrii [3], and R. philippinarum and Nuttallia olivacea for A. macracanthum [5]. In the present study, M. veneriformis has been confirmed to be a second intermediate host for A. mariale. It is of note that M. veneriformis is also known to be a second intermediate host for a gymnophallid fluke, Parvatrema chaii [16].
Among the human-infecting echinostomatid flukes, A. tyosenense is the only known species belonging to Acanthoparyphium. It has been suggested that human infections with A. tyosenense were contracted by consumption of the raw flesh of intertidal bivalves, including M. veneriformis [7]. Therefore, attention should be focused on the possible presence of human infections with A. marilae in the Republic of Korea.
ACKNOWLEDGEMENTS
The authors wish to thank Jae-Lip Kim for his excellent technical assistance.