Sparganosis Presenting as Cauda Equina Syndrome with Molecular Identification of the Parasite in Tissue Sections
Article information
Abstract
A 52-year-old woman presented with lower back pain, progressive symmetrical paraparesis with sensory impairment, and sphincter disturbance. Magnetic resonance imaging (MRI) of the whole spine revealed multiple intradural extramedullary serpiginous-mass lesions in the subarachnoid space continuously from the prepontine to the anterior part of the medulla oblongata levels, C7, T2-T8, and T12 vertebral levels distally until the end of the theca sac and filling-in the right S1 neural foramen. Sparganosis was diagnosed by demonstration of the sparganum in histopathological sections of surgically resected tissues and also by the presence of serum IgG antibodies by ELISA. DNA was extracted from unstained tissue sections, and a partial fragment of mitochondrial cytochrome c oxidase subunit 1 (cox1) gene was amplified using a primer set specific for Spirometra spp. cox1. After sequencing of the PCR-amplicon and alignment of the nucleotide sequence data, the causative agent was identified as the larva of Spirometra erinaceieuropaei.
INTRODUCTION
Human sparganosis is a zoonosis caused by plerocercoid larvae (=spargana) of pseudophyllidean (now classified as diphyllobothriidean [1,2]) tapeworms [3,4]. In Asia, S. erinaceieuropaei is responsible for this disease, whereas Spirometra mansonoides is important in the Americas. Sparganosis usually appears as slowly growing and migratory subcutaneous nodules. This parasite can be found anywhere in the body including the central nervous system [5,6]. In rare cases, a sparganum involves the spinal cord, usually at the thoracic to lumbar levels [7-15]. To our knowledge, only 3 cases have been reported as sparganosis with cauda equina syndrome [11,14,16]. None of these cases were confirmed by molecular diagnosis. Here, we describe a case of cauda equina syndrome with molecular evidence for identification of the causative agent and review the literature of sparganosis involving the spinal cord.
CASE RECORD
The patient was a 52-year-old female sugarcane farmer who lived in a rural community of Suphan Buri Province, central Thailand. She presented to our hospital complaining of lumbodorsal pain for the previous month, progressive symmetrical paraparesis with sensory impairment and radiculopathy for 2 weeks, and bowel/bladder dysfunction for 3 days. She had no history of ingesting inadequately cooked frogs, snakes, or birds. On examination, the patient had a normal body temperature. Skin nodules or organomegaly was not observed. Mental status and cranial nerve functions were within normal limits. Neurological examinations revealed decreased motor tone of both legs. Motor strength of the left lower extremity decreased from grade V (normal) to grade III in the hamstrings, iliopsoas, and quadriceps muscles; grade I-II in the ankle and toe plantar flexor muscles; and grade 0 in the ankle dorsiflexors muscles and the extensor hallucis longus muscle. For the right lower extremity, motor strength was decreased to grade III in all the muscles. Sensations were decreased below the L2 dermatome on both sides with peri-anal anesthesia, and the knee and ankle jerk were absent on both sides. Anal sphincter tone was reduced. A clinical diagnosis of cauda equina syndrome was made. Routine biochemical and hematological investigations were within normal limits except for an increase of absolute eosinophil count in the peripheral blood (0.46×109/L). MRI of the whole spine showed multiple intradural extramedullary serpiginous-mass lesions in the subarachnoid space continuously from the prepontine to the anterior part of the medulla oblongata levels, C7, T2-T8, and T12 vertebral levels distally until the end of the thecal sac, and filling-in of the right S1 neural foramen (Fig. 1). Attempts of spinal tapping to obtain a cerebrospinal fluid (CSF) sample were unsuccessful.
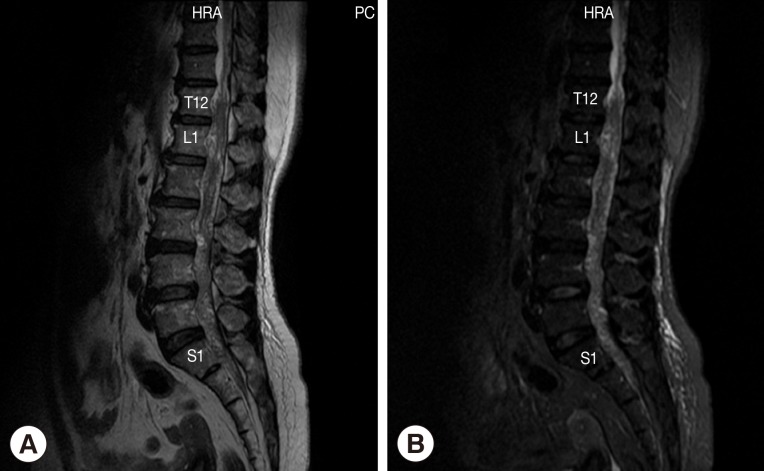
Magnetic resonance of lumbosacral spine sagittal T2W image (A) and sagittal T2W with fat suppression image (B) shows abnormal lower lumbar spinal cord, conus medullaris, and cauda equina. Multiple heterogeneously serpigineous lesion filling within T12 to S1 of the thecal sac.
Laminectomy at L1 and L2 level was performed to obtain a tissue sample for histopathological examinations. During the operation, we noticed a matted mass involving nerve roots with remarkable inflammation and multiple cystic lesions containing pus; no CSF was seen due to obstruction by the mass. A small piece of tissue from the matted mass was removed and fixed in formalin and processed for paraffin embedding. The histological sections were stained with hematoxylin and eosin. Histological examinations demonstrated a degenerated solid section of a folded cestode larva having irregular folding tegument which was homogenously eosinophilic in color. Many calcareous corpuscles (Fig. 2B arrows), which is a characteristic feature of a cestode larva, were seen. There were mild inflammatory cell infiltrations including eosinophils, lymphocytes, a few plasma cells, and neutrophils in the larval section (Fig. 2). The definite diagnosis of sparganosis could be made from the histopathological findings.
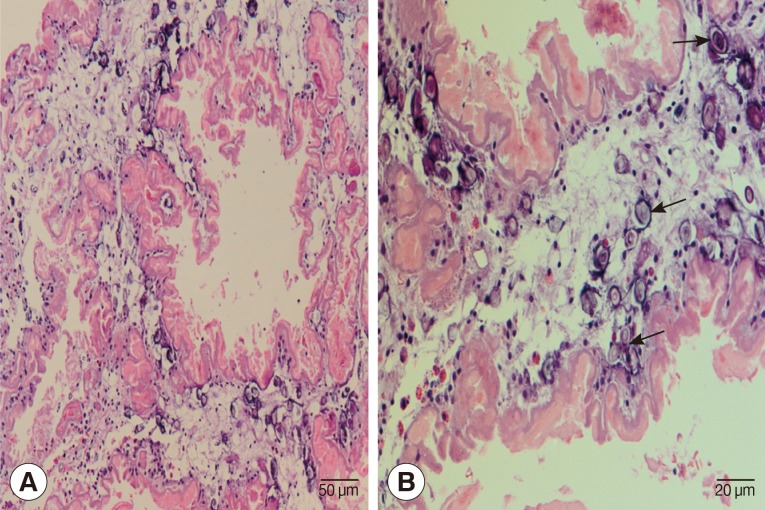
Histopathological findings of the resected lesion. (A) Section of a degenerating cestode larva (molecular confirmed as Spirometra erinaceieuropaei) with homogenously eosinophilic and irregularly ridged tegument. Calcareous bodies and inflammatory cell infiltrations are seen throughout the parasite body. ×200. (B) Calcareous bodies (arrows) and mild inflammatory cell infiltrations including lymphocytes and a few eosinophils, plasma cells, and polymorphonuclear cells are seen in the body of the cestode larva. ×400.
After the histopathological confirmation, serological tests for detection of specific antibodies in the serum was performed. Using ELISA, we detected a high titer of specific IgG antibodies against S. erinaceieuropaei sparganum partially purified antigen. The serum was negative for cysticercosis by ELISA and also negative for gnathostomiasis, paragonimiasis, fascioliasis, and angiostrongyliasis by immunoblotting.
For molecular identification of the causative parasite species, DNA was extracted from 10 µm unstained serial sections (cut from the formalin-fixed paraffin-embedded specimen) attached to glass slides using a DEXPAT kit (TaKaRa Bio Inc., Tokyo, Japan) as reported previously [17]. The resulting supernatants were used as the DNA template for PCR. Amplification of mitochondrial cytochrome c oxidase subunit 1 (cox1) gene by PCR was performed in a 25 µl reaction mixture. A fragment of cox1 gene was amplified using the primers Se658-F (5'-TTT GAT CCT TTG GGT GGT GG-3') and Se1124-R (5'-ACC ACA AAC CAC GTG TCA TG-3'), which were designed from the cox1 gene of S. erinaceieuropaei (GenBank accession no. AB369250). PCR was carried out using a GeneAmp PCR System 9700 (Applied Biosystems, Singapore). The reaction was carried out in a 25 µl volume containing 2.5 µl of 10x FastStart High Fidelity Reaction buffer with 18 mM MgCl2 (Roche, Mannheim, Germany), 200 µM of each deoxyribonucleotide triphosphate, 0.2 µM of each primer (Invitrogen, Carlsbad, California, USA), and 0.625 units of FastStart High Fidelity Enzyme Blend (Roche). The DNA template was initially denatured at 94℃ for 5 min. The amplification procedure consisted of 35 cycles at 95℃ for 30 sec (denaturation), 59℃ for 30 sec (annealing), and 72℃ for 45 sec (extension), with a final extension at 72℃ for 10 min. Amplified product was run on a 1% agarose gel; a 467 bp fragment was cut and sequenced using the MegaBACE™ 1000 DNA Analysis System (GE Healthcare, Piscataway, New Jersy, USA). The cox1 gene sequence of S. erinaceieuropaei obtained from the patient was analyzed by BLAST-N search via NCBI.
The partial cox1 sequence of S. erinaceieuropaei from the patient, which was deposited in the GenBank database (no. KC551943), was almost completely (97-99% identity) identical with those of S. erinaceieuropaei from various geographical localities. From these results, the parasite obtained from the patient was identified as S. erinaceieuropaei.
After laminectomy at L1 and L2 level, the patient received corticosteroid and praziquantel therapy, and her lumbosacral pain moderately improved. However, she persistently complained of a dull, uncomfortable sensation on both buttocks. At follow-up after 1 month, paraparesis with sensory impairment, urination, and defecation difficulties still persisted. This study protocol was approved by Siriraj Institutional Review Board Certificate of Approval (COA no. Si 189/2012).
DISCUSSION
Sparganosis is an uncommon disease in humans. It is caused by the larvae of the tapeworm genus Spirometra, whose definitive hosts are domestic and wild cats and dogs [11,14,16]. Human infections usually occur in the following 3 ways; drinking untreated water containing infected copepods; ingesting raw or inadequately cooked flesh of snakes, frogs, or birds infected with spargana; and applying the flesh of an infected frog as a poultice to a wound [18]. The route of infection of our patient remains uncertain. The disease usually involves the subcutaneous tissue or muscles of the chest, abdominal wall, or limbs. Central nervous system involvement is relatively rare, involvement of the spinal cord, in particular, is extremely rare [5,19]. In the literature [7-10,12-16], including the present study, 10 cases of spinal sparganosis have been reported. These were 6 males and 4 females whose ages ranged from 10 to 59 years (mean age; 38.9 years). The sparganum more commonly involved intradural (70%) than extradural [8,9,15] sites, usually at the thoracic level [7-10,12,13], followed by the cervical level [9]. Including the present case, there were only 3 cases of sparganosis involving the conus medullaris and cauda equina [13,14]. Our patient demonstrated long and multiple intradural extramedullary lesions extending from the prepontine to the anterior part of medulla oblongata levels, C7, T2-T8, and T12 vertebral levels distally until S1 level. Clinical manifestations included sensory disturbances (70%), weakness of the limbs (60%), pain (60%), and voiding difficulty (50%). The mean duration of symptoms before diagnosis was 9.4 months (range; 3 days to 3 years). All patients received surgical treatment; our patient received postoperative medical treatment. The prognosis was good or fair.
In nearly all of the spinal sparganosis patients, lesions were detected in the thoracic cord. Our patient was unique in that the lesions were at multiple levels from the prepontine down to the end of the thecal sac. The patient presented with severe lumbodorsal pain due to a mass compressing the adjacent spinal cord and resulting spinal cord edema. Pain in the nerve root may occur long before the signs of spinal cord compression have developed. In our patient, lumbodorsal pain developed before paraparesis and bowel/bladder involvement. The route of the entry of worms into the spinal cord remains unclear, but hematogenous spread seems likely.
It is hard to diagnose sparganosis clinically and to differentiate it from neoplastic and inflammatory disorders or other parasitic visceral larva migrans because it is rare and has non-specific manifestations. We could not find any specific features for diagnosis in neuroradiological imaging of these cases. An immunological approach using ELISA is presently used for diagnostic purpose. ELISA for detecting anti-sparganum IgG antibody is highly sensitive (85.7-100%) and specific (95.7%) [20]. However, the ELISA technique can not identify the causative worm at the species level. PCR-based molecular techniques should be used for identification of the causative pathogens of infectious diseases. Recent development of PCR/sequencing technique for DNAs from formalin-fixed paraffin-embedded (FFPE) tissues kept for many years enabled us to perform the retrospective re-appraisal of individual cases and also epidemiological studies [21].
In conclusion, we report here a rare case of sparganosis presented as cauda equina syndrome. PCR-based identification of the causative agent using FFPE tissues led us to the accurate and definite diagnosis.
ACKNOWLEGMENTS
This study was supported by TRF Senior Research Scholar Grant, Thailand Research Fund grant number RTA5580004 to Pewpan M. Intapan and Wanchai Maleewong and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission through the Health Cluster (SHep-GMS) and Khon Kaen University, Khon Kaen, Thailand.