Prevalence and Clinical Manifestations of Malaria in Aligarh, India
Article information
Abstract
Malaria is one of the most widespread infectious diseases of tropical countries with an estimated 207 million cases globally. In India, there are endemic pockets of this disease, including Aligarh. Hundreds of Plasmodium falciparum and P. vivax cases with severe pathological conditions are recorded every year in this district. The aim of this study is to find out changes in liver enzymes and kidney markers. Specific diagnosis for P. falciparum and P. vivax was made by microscopic examination of Giemsa stained slides. Clinical symptoms were observed in both of these infections. Liver enzymes, such as AST, ALT, and ALP, and kidney function markers, such as creatinine and urea, were estimated by standard biochemical techniques. In Aligarh district, P. vivax, P. falciparum, and mixed infections were 64%, 34%, and 2%, respectively. In case of P. falciparum infection, the incidences of anemia, splenomegaly, renal failure, jaundice, and neurological sequelae were higher compared to those in P. vivax infection. Recrudescence and relapse rates were 18% and 20% in P. falciparum and P. vivax infections, respectively. Liver dysfunctions and renal failures were more common in P. falciparum patients, particularly in elderly patients. Artesunate derivatives must, therefore, be introduced for the treatment of P. falciparum as they resist to chloroquine as well as sulfadoxine-pyrimethamine combinations.
INTRODUCTION
Malaria is endemic in 104 countries with estimated 207 million clinical cases and 627,000 deaths, representing a major global public health problem [1]. It mainly affects children, pregnant women, and non-immune adults who frequently die as victims of cerebral manifestations and anemia [2]. Both of these malarial complications are responsible for a great number of spontaneous abortions, stillbirths, premature deliveries, and low birth weight [3]. Children with impaired consciousness and respiratory distress are at the highest risk of death associated with malaria [4]. Among the 4 Plasmodium species responsible for human malaria, P. falciparum has been the most prevalent overall, particularly in Africa. Neurological involvement in P. falciparum malaria is common and nearly a quarter of children who survived cerebral malaria develop neurological sequelae [5]. P. vivax is responsible for a significant proportion of malaria cases worldwide and is increasingly reported as a cause of severe disease [6]. In the past few years, P. vivax infection was recognized as a cause of severe malaria in young children even causing death [7,8,9].
In India, 60-65% of the malaria infections are reported due to P. vivax, while 30-35% due to P. falciparum [10]. India is one of the major contributors to malarial morbidity and mortality in the world [11]. Malaria has been a serious problem in some parts of India due to the slow progress in its control. During the past decade, most malaria-endemic countries shifted their national treatment policies to artemisinin combination therapy (ACT). Keeping above facts in view, the present study aimed to determine the prevalence and clinical manifestations of malaria in Aligarh, India.
MATERIALS AND METHODS
This study is based on the confirmed cases of malaria admitted in the hospitals and nursing homes of Aligarh, India during 2011-2013. Blood samples were collected from the patients admitted to the Jawaharlal Nehru Medical College hospital and a few other nursing homes of Aligarh. Clinical profiles of the patients were recorded at the time of sample collection. Patients having symptoms resembling malaria because of any other infections were excluded from the study. Thin and thick blood smears were prepared on the glass slides by pricking the finger of the patients complaining for high fever and headache. Thin films were fixed with methanol before staining with Giemsa. For specific diagnosis of Plasmodium falciparum and Plasmodium vivax, slides were examined by Nikon Eclipse-600 research microscope at ×100. Rapid diagnostic test kits were also used during outdoor collections. Infectivity was recorded for these 2 species and mixed infections by observing typical ring stages and gametocytes.
For liver and kidney function tests (LFT and RFT), blood was obtained after taking patients' consent by vein puncture using a 21 gauge hypodermic sterile needle and syringe. These samples were collected in a clean sterile glass tubes, allowed to clot for 2 hr at room temperature, and then in a refrigerator at 5℃ for 10 hr so that clot could get squeezed. Serum was picked and transferred to centrifuge tubes with the help of pipette and centrifuged at 3,000 rpm for 5 min to remove any cell contamination. The sera were then stored in separate vials for biochemical assay at -20℃ until analyzed for liver enzymes and kidney markers. Changes in liver enzymes, such as aspartate transaminase (AST), alanine transaminase (ALT), and alkaline phosphatase (ALP), urea, and creatinine of the patients were estimated with the help of standard techniques. AST and ALT were estimated as described by Wilkinson et al. [12]. ALP was measured by the method of Burtis and Ashwood [13]. Kidney function markers, such as creatinine and urea, were estimated according to the procedures described by Thomas [14] and Kaplan [15], respectively. Symptomatic and pathological findings were presented in the form of tables and figures. Clinical symptoms, such as high fever, chill, vomiting, anemia, splenomegaly, jaundice, respiratory distress, convulsions, and neurological sequelae were recorded in P. falciparum, P. vivax, and mixed infections.
Statistical analysis
Statistical analysis was performed using SPSS 16.0 (SPSS Inc. Chicago, Illinois, USA). All the clinical variables were examined using the chi-square test, and the level of significance was set at P<0.05. The results were expressed as mean±SD. Graphs were plotted on Microsoft excel sheets.
RESULTS
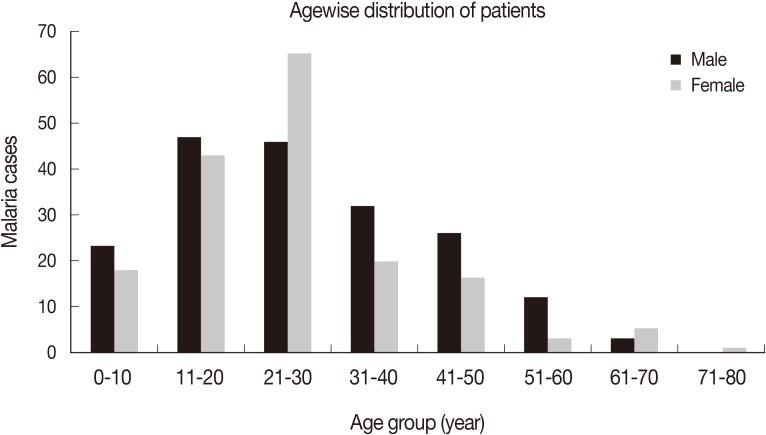
A total of 360 cases of malaria patients were observed during 2011-2013 from Aligarh, India. Among them, 64% (n=231) belonged to P. vivax, 34% (n=122) to P. falciparum, and 2% (n=7) were the cases of mixed infection. All the patients had fever which ranged from 101 to 105°F (38-41℃), while chill was observed in about 80% cases. Other prominent symptoms observed were sweating, headache, nausea, dizziness, and joint pain in many of the patients (Table 1). A few resistant and relapsing cases of P. falciparum and P. vivax were also recorded during the study. When treated with 1,500 mg chloroquine in divided doses for 3 days, the resistance rate in falciparum malaria was 18%, while the relapse rate was 20% in P. vivax malaria patients. The majority of the relapse cases (15%) were of short term. The maximum numbers of malaria patients were recorded from the age group of 21-30 years, followed by 10-20 years (Fig. 1). Generally, male patients dominated in almost all age groups except 21-30 years where females were more in number.
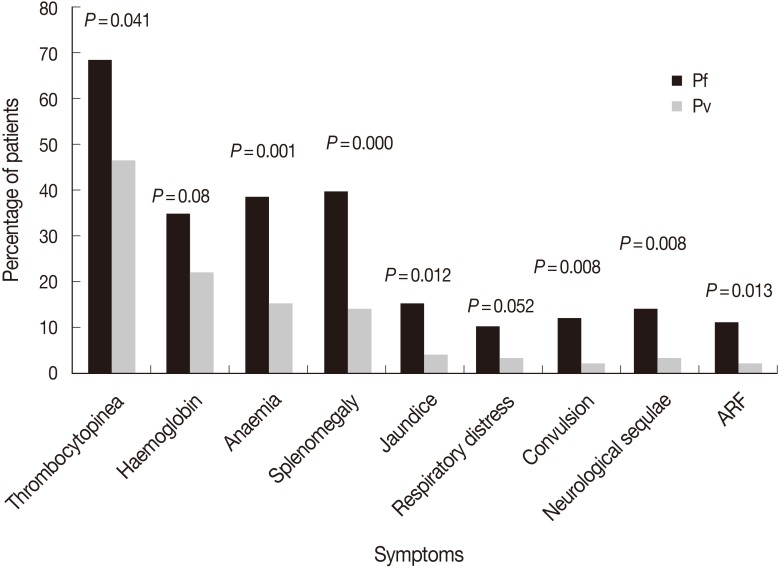
Pathological findings of both P. falciparum and P. vivax are summarized in Fig. 2. There were contrasting differences with respect to thrombocytopenia, jaundice, respiratory distress, convulsions, neurological sequelae, and acute renal failures in terms of percentage which were much higher in P. falciparum infections (69, 15, 10, 12, 14, and 11%, respectively) compared to P. vivax where the percentage of these parameters were much less (47, 4, 3, 2, 3, and 2%, respectively). Except for hemoglobinemia, other parameters showed significant differences (P<0.05). Hemoglobinemia was more prominent in P. falciparum patients (35%) than in P. vivax malaria patients (22%). Thirty-nine percent of P. falciparum patients were anemic compared to 15% of P. vivax patients. Anemia was rarely observed in healthy individuals or patients having mild infections. Splenomegaly was much pronounced and was observed in 40% of P. falciparum infections compared to P. vivax, where it was only in 15% cases.
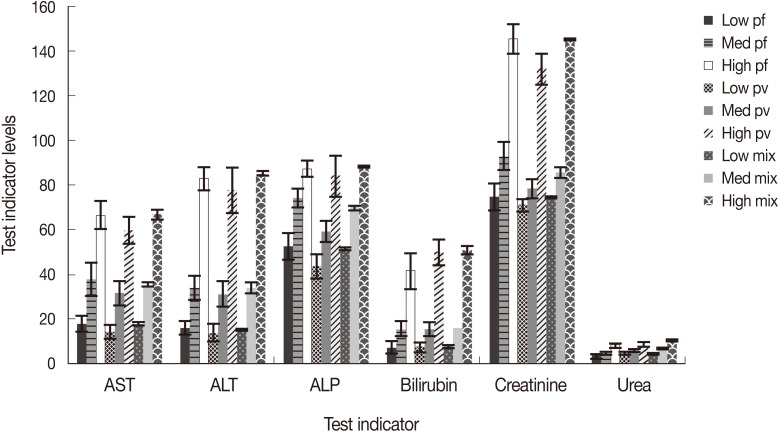
Liver dysfunction is one of the causes in severe malaria patients which aggravate sickness. Liver function test (LFT) was performed by estimating serum bilirubin, AST, ALT, and ALP. The technique applied for LFT had normal values of 0-45, 3-60, and 30-71 IU/L for AST, ALT, and ALP, respectively, while it was 3-20 µmoles/L for serum albumin. Liver enzymes were elevated in severe cases especially in falciparum malaria where the parasite density was high (>10 parasites/field). AST, ALT, and ALP showed higher values (66.4±6.1, 82.9±5.0, 87.1±3.5, respectively) in severe P. falciparum cases, while it was slightly less in P. vivax cases (59.7±6.1, 77.6±10.1, 84.0±9.3, respectively) and high (66.5±2.0, 84.9±1.0, 88.1±0.4, respectively) in cases of mixed infections having both P. falciparum and P. vivax. Serum bilirubin was also raised and ranged between 41.4±7.8 and 50.6±1.9 µmoles/L in severe malaria cases irrespective of the species of malaria (Fig. 3). Normal range was observed in the level of enzymes in all malaria patients showing low and mild infections.
In severe malaria patients where treatment was delayed especially in P. falciparum, there were indications of renal impairments in the form of raised creatinine and urea values. Patients with high parasitemia showed an increase in the level of creatinine at 145.0±6.5, 131.8±7.1, and 141.5±0.3 µmoles/L in cases of P. falciparum, P. vivax, and mixed infections, respectively, compared to normal ranges of 72-126 µmoles/L. Serum urea also showed slightly higher values (7.9±1.0, 8.4±0.8, and 10.4±0.2, respectively) against the normal range of 3.0-6.0 mmoles/L (Fig. 3).
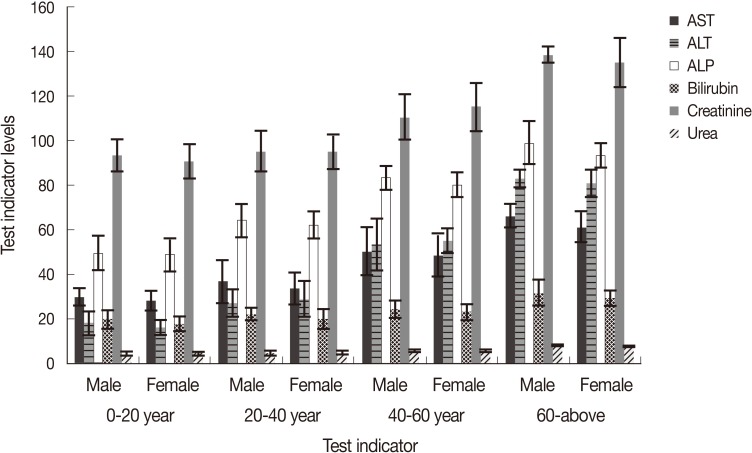
In P. falciparum, age and sex of the patients showed variations in the level of liver enzymes. Elderly people between 40-60 years showed elevated values for AST, ALT, and ALP which ranged 49.6-66.5, 52.8-85.9, and 83.0-101.2 IU/L, respectively, compared to younger patients where values for these enzymes were within normal ranges (26.3-33.9, 17.6-33.3, and 50.9-66.0 IU/L, respectively, Fig. 4). Serum bilirubin was also raised in older patients (29.8-35.8) compared to younger ones where values were almost in normal range (15.0-21.4). In most of the patients, creatinine and urea were found to be in their normal ranges excepting in older patients who showed a little higher levels of 139.6 µmoles and 8.6 mmoles/L, respectively. Almost similar trend was seen in the liver enzymes and kidney markers in P. vivax patients, where elevated values were recorded in severely affected elderly patients (Fig. 5). In P. vivax, highest values of AST, ALT, ALP, and serum bilirubin were 66.2, 82.9, 98.9 IU/L, and 31.8 µmoles/L, respectively. Creatinine and urea were also slightly raised in older patients and were 139.4 µmoles/L and 8.2 mmoles/L, respectively. Generally, male patients showed slightly higher levels of liver enzymes as well as kidney markers in almost all age groups (Figs. 4, 5).
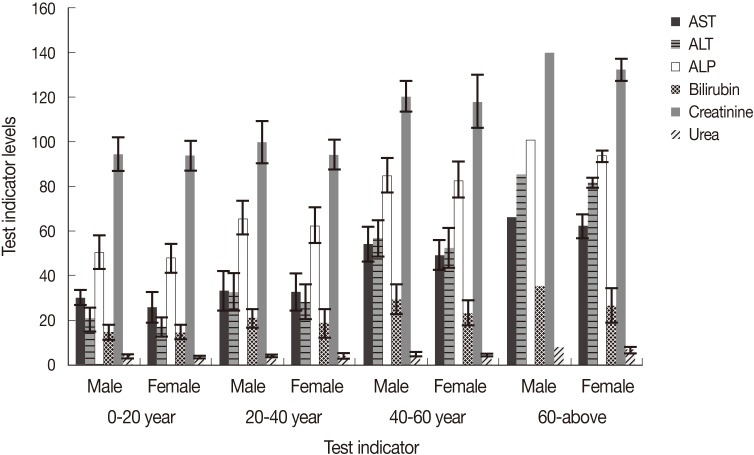
Liver enzymes, serum bilirubin, creatinine, and urea in different age groups of male and female patients of P. falciparum.
DISCUSSION
We diagnosed 360 malaria patients between 2011 and 2013. The most frequently implicated species was P. vivax, accounting for 64% of cases, while P. falciparum accounted for 34%. The results are in accordance with the earlier findings in which P. vivax and P. falciparum were reported to be 56.5 and 39.1%, respectively, in Central India [16]. This indicates that P. vivax is the most widespread infection in India which results in a pronounced morbidity [17]. Recently, it had been reported that P. vivax also has immense potential to cause life threatening complications and even death [18]. During our study, we reported 20% relapse cases in vivax malaria. Much higher relapse rates ranging from 23% to 44% depending on the duration of follow-up which lasted for 5 years was reported from Delhi [10]. This difference might be due to a more number of cases observed in Delhi for a longer duration, but a possibility of including reinfection cases cannot be ruled out. In order to reduce relapsing cases, 15 mg primaquine, once a day for radical cure of P. vivax infection, needs its reconsideration, especially in areas where chloroquine is still given as the first line of treatment. We noticed positive slides during the follow up in 18% P. falciparum patients which were treated with 1,500 mg chloroquine. Resistance rates ranging from 25-30% were reported earlier from high P. falciparum transmission zones of India [19]. Low percentage of resistant cases in our study area might be due to that it is a low transmission region where P. falciparum infection is less compared with P. vivax.
As far as clinical symptoms are concerned, all the patients had high fever with chills in 80% of them as earlier observed in Pakistan [20]. Other symptoms, such as headache, sweating, nausea, and joint pain were slightly high in P. falciparum cases compared to P. vivax cases. Almost similar clinical patterns for most of the above parameters were observed in severe malaria cases in Jazan [21]. In the present study, cases of P. vivax, P. falciparum, and mixed infections were observed in 64%, 34%, and 2%, respectively which is almost similar to an earlier study conducted in Karnataka where P. falciparum and P. vivax were 53 and 34%, and mixed infections were 14% [22]. Species composition varies even in the same country having different ecological conditions with different types of breeding places and vectors. The agewise distribution showed that the maximum number of patients were in the age group 21-30 years followed by 11-20 years, indicating that this is most active age in which maximum exposure could take place. This explanation may support higher percentage in males of all age groups excepting 21-30 years where females dominated which could not be explained. One of the earlier studies showed a maximum number of cases in the patients of 21-30 age groups as our results, but with much difference in percentages (i.e., 81 and 19%) for males and females, respectively [22].
In the present study, we observed thrombocytopenia in 69% of P. falciparum and 47% of P. vivax cases. From another part of India, thrombocytopenia was reported in 50% of P. falciparum and 65% of P. vivax patients [23], which are just reverse to our values. Slightly lower levels of thrombocytopenia, 54.5% and 29.5%, was reported for P. falciparum and P. vivax infections, respectively, in Pakistan [20]. Variations in values might be due to drug resistance and endemicity, as drug resistant parasites prolong their stay in their host and do more damages resulting in thrombocytopenia [24]. The elevated figure of thrombocytopenia in P. vivax infection showed that this species is also moving towards malignancy.
Malaria is a true hematological disease affecting almost all blood components. Anemia is an inevitable consequence in malaria as Plasmodium-invaded red blood cells lead to rigidity and become adherent. The intracellular parasites modify the erythrocytes in several ways and cause hypoglycemia by converting glucose into lactic acid by anerobic glycolysis [25]. The cell membranes become less deformable, resulting in hemolysis and anemia. Alteration to uninfected red blood cells by addition of glycosylphosphatidylinositol (GPI) to membrane causes increased clearance of cells in P. falciparum contributing to anemia [26]. We observed anemia in 39% of P. falciparum and 15% of P. vivax cases, considering WHO criteria for severe malaria which is <7 g/dl for adults and <5 g/dl for children. Higher rates of anemia were recorded in P. falciparum (60%) and P. vivax (19%) patients of Saudi Arabia and Indonesia [24,27]. In contrast, much lower counts for anemia of around 13% and 3% for P. falciparum and P. vivax, respectively, were recorded from Mumbai [9]. This variation might be due to the severity of infection and the level of immunity against the parasite in patients of falciparum and vivax malaria in different countries having endemic and non-endemic pockets.
Generally, spleen enlarges more in P. falciparum infections due to phagocytosis of parasitized RBC and their accumulation in spleen for clearance. We observed splenomegaly in 40% of P. falciparum which was detected only in 14% of P. vivax patients. An almost similar enlargement of around 39-45% was observed P. falciparum patients of Saudi Arabia [21,28]. However, much higher rate of splenomegaly (71%) was recorded in falciparum malaria from the other parts of India [29]. These variations might be due to differences in the immune status of patients from different malaria transmission regions.
As far serum bilirubin is concerned, we observed elevated values of 41.4±7.8, 49.9±5.7, and 50.6±1.9 µmoles/L in patients severely infected with P. falciparum, P. vivax, and in mixed infections, respectively, which is well supported by earlier findings obtained in Karnataka where a similar elevated level of bilirubin was recorded in 46% of P. falciparum infections [30]. However, bilirubinemia was noticed in 32%, 9%, and 49% of P. falciparum, P. vivax, and mixed infection cases in Pakistan [20]. In Mumbai, bilirubinemia was recorded in 22% of P. falciparum and 5% of P. vivax patients [9], which are relatively low counts compared to our study. We observed 15% of patients with jaundice in P. falciparum infections in Aligarh which was reported as 20% and 40% in patients of Orissa and Calcutta [31,32]. We observed jaundice in 4% of P. vivax patients from our study area. Higher rates of 9.0-9.5% were recorded by other workers in India and Pakistan [20,32]. Since jaundice was mostly noticed in severe cases of P. falciparum, P. vivax, and mixed infections, it appears that jaundice is an outcome of combined effects of hemolysis, hyperbilirubinemia, and liver dysfunction.
In our study, it was observed that mean AST, ALT, and ALP values were 66.4±6.1, 82.9±5.0, and 87.1±3.2 in the P. falciparum cases, while 59.7±6.1, 77.6±10.1, and 84.0±9.3 in P. vivax, respectively. These enzymes were also above the normal range in mixed infections (i.e., 66.5±2.0, 84.9±1.0, and 88.1±0.4, respectively). Increased liver enzymes may result in liver dysfunction in a few patients having high parasitemia. Those who had mild and low parasitemia, the levels of enzymes were within normal ranges. Comparatively lower levels of AST, ALT, and ALP (33.8, 34.8, and 91.4, respectively) were recorded for mild infections in Nigeria [33]. Elevated levels of AST, ALT, and ALP (75, 162, and 265 IU/L) were also reported for P. vivax patients from New Delhi, India [34]. There was a significant rise in the level of liver enzymes in patients with high parasitemia as compared to those having mild and low infections (Fig. 3). Levels of liver enzymes were higher in elderly patients compared to younger ones (Figs. 4, 5). This shows that the parasites during their hepatic phases destroy the membrane of parasitized cells leading to leakage of the liver enzyme into the blood circulation. Injury of the cells in elder patients might be due to the abnormal cell activations and autoimmune progress induced by the parasites especially when parasitemia was high, while younger patients who were less exposed, injury to hepatocytes were comparatively less. However, damage of the hepatocytes and leakage of enzymes due to the toxicity of antimalarials administered to treat malaria cannot be ruled out.
Acute renal failure is a common cause of morbidity and mortality in severe malaria patients of Southeast Asia and Indian subcontinent where transmission rate is low but there are occasional burst in malaria transmission in small pockets during post monsoon, resulting in an increased creatinine level in P. falciparum and P. vivax patients having high parasitemia. In the present study, creatinine was recorded more than that of the normal range in patients suffering from both falciparum and vivax malaria. We observed acute renal failures (ARF) in 11% of P. falciparum and 2% of P. vivax patients from our study area. Comparatively much higher rates of ARF of 19 and 21% were recorded from the patients of other parts of Pakistan, India, and Thailand [31,35,36]. We observed a comparatively low rate of ARF in 2% of P. vivax infections in Aligarh, while earlier workers reported little higher values of 2.5 and 3.5% ARF from Pakistan and India, respectively [37,38]. Unusually much higher renal failures were recorded in the range of 12.5% and 20.0% in P. vivax infections in other high transmission regions of India [39,40]. Usually ARF occurs in non-immune individuals especially in P. falciparum infections with high parasitemia, but we noticed renal failures in P. vivax infections as well which was generally considered as benign infections during the past warrants immediate attention and change in treatment strategy. Physiological changes especially in falciparum malaria transforms infected erythrocytes which make them adherent to each other as well as walls of capillary vessels which results in mechanical obstructions. Besides, immune-mediated glomerular pathology by the circulating parasites which inflames and alters renal microcirculation by the adherent RBCs along with increased harmful substance like creatinine in the blood which damage the kidney tissue, might be the probable cause of renal failures [41], which also seems true in our case. Slightly raised levels of urea were recorded (7.9-10.5 mmoles/L) in high parasitemia patients with severe P. falciparum and P. vivax malaria.
We observed acute respiratory distress (ARD) in 10% of P. falciparum and 3% of P. vivax cases. In other reports of India, ARDs were reported in 6.6% in P. falciparum and 9.7% in P. vivax infections [18,32]. However, in both infections, this phenomenon occurs as parasites derive energy from anerobic glycolysis of glucose which yields lactic acid, which may contribute to clinical manifestations of hypoglycemia and lactic acidosis leading to respiratory distress in a few severely infected patients.
Neurological syndrome manifests as impaired consciousness and convulsions which often leads to coma. We observed neurological syndrome in 14% of P. falciparum patients but none in coma. In other studies, 12-21% patients were reported to have convulsions and other neurological deficits after surviving cerebral malaria [42,43]. We observed convulsion in 2% of P. vivax infection. Low rates of convulsions and other neurological disturbances from our study area might be due to occurrence of low and mild infections in majority of the cases.
From the results obtained during the present study, it may be surmised that thrombocytopenia, anemia, splenomegaly, jaundice, and neurological sequelae were more common in patients having severe P. falciparum infection. However, a few patients of P. vivax with high parasitemia also had aforesaid complication which is unusual for this species. Hepatic dysfunction and renal impairments noted in P. vivax is a matter of concern as it was presumed to be a benign species. In order to avoid further complications in malaria, it is suggested that both P. falciparum and P. vivax infections must be treated with the most effective antimalarials, preferably combined therapy aiming at different biochemical targets.
Notes
We have no conflict of interest related to this work.
ACKNOWLEDGMENTS
This work has been supported by MANF scholarship programme of University Grants Commission, New Delhi, India. Mrs. Umm-e Asma and Farha Taufiq are in receipt of senior research fellowships vide grant nos. F.406-3 (M/S)/2009 (SA-III/MANF) and F1-17-1/2011/MANF-MUS-UTT-889/(SA-III/Website) which is thankfully acknowledged. The authors also wish to thank Dr. Shabi Fatima Abidi for critical review of this manuscript.