Toxoplasma gondii B1 Gene Detection in Feces of Stray Cats around Seoul, Korea and Genotype Analysis of Two Laboratory-Passaged Isolates
Article information
Abstract
The increasing prevalence of Toxoplasma gondii infection in the human population in the Republic of Korea (= Korea) is due to various reasons such as an increase in meat consumption. However, the importance of cats in transmitting T. gondii infection through oocysts to humans has seldom been assessed. A total of 300 fecal samples of stray cats captured around Seoul from June to August 2013 were examined for T. gondii B1 gene (indicating the presence of oocysts) using nested-PCR. Fourteen (4.7%) of 300 cats examined were positive for B1 gene. Female cats (7.5%) showed a higher prevalence than male cats (1.4%). Cats younger than 3 months (5.5%) showed a higher prevalence than cats (1.5%) older than 3 months. For laboratory passage of the positive samples, the fecal suspension (0.2 ml) of B1 gene positive cats was orally inoculated into experimental mice. Brain tissues of the mice were obtained after 40 days and examined for the presence of tissue cysts. Two isolates were successfully passaged (designated KNIH-1 and KNIH-2) and were molecularly analyzed using the SAG5D and SAG5E gene sequences. The SAG5D and SAG5E gene sequences showed high homologies with the ME49 strain (less virulent strain). The results indicated the importance of stray cats in transmitting T. gondii to humans in Korea, as revealed by detection of B1 gene in fecal samples. T. gondii isolates from cats were successfully passaged in the laboratory for the first time in Korea.
INTRODUCTION
Toxoplasma gondii is an intracellular protozoan parasite that can infect warm-blooded animals, including humans. Toxoplasmosis is an important clinical disease worldwide and can lead to lymphadenitis, encephalitis, retinochoroiditis, congenital infection, and stillbirth [1]. T. gondii can cause infection via ingestion of tissue cysts or tachyzoites in raw or undercooked meat of infected animals, or ingestion of oocysts in the water or soil contaminated with feces of infected cats [2].
The prevalence of human toxoplasmosis in the Republic of Korea (=Korea) is increasing due to various factors. Increase in meat consumption is an important reason. Also, individuals with occupations requiring soil contact in environments frequented by cats are significantly more likely to contract toxoplasmosis [2]. However, a more significant risk factor is direct contact with cats, the definitive host of T. gondii. Epidemiologic surveys of T. gondii infection in sera of stray and household cats have been reported in several areas of Korea [3-8].
T. gondii strains isolated from Europe and North America belong to 3 distinct clonal lineages (genotypes I, II, and III) that differ in phenotype, including the pathogenicity [9]. Dubey et al. [10] recently found the 4th clonal lineage (genotype 12) from wild life of North America. They found that 85% of different strains in North America were 1 of the 3 widespread genotypes including genotype II, genotype III, and genotype 12 [10]. With the exception of a few reports, there has been little information about T. gondii strains and isolates in Korea. Only 1 long-term laboratory-passaged Korean isolate (KI-1) that was originally isolated from an ocular toxoplasmosis patient is available [11]. The gene sequences of KI-1 were highly homologous with RH, and thus KI-1 has been included in the genotype I [12]. In addition, sequence polymorphisms and phylogenetic characteristics were studied on T. gondii genes from heart tissues of small mammals captured in Gyeonggi and Gangwon Provinces of Korea; they closely aligned with the genotype I [13].
To date, there have been few surveys on the prevalence of T. gondii in stray cats living around Seoul, Korea, particularly using fecal samples to detect B1 gene. Characterization of new geographical strains or isolates of T. gondii is also needed in Korea. Thus, we performed a brief survey of T. gondii infection in stray cats captured around Seoul, Korea by means of nestedPCR to detect B1 gene and conducted laboratory-passage of some of the isolates for determination of the genotype.
MATERIALS AND METHODS
Sample collection
Fecal samples were collected from 300 stray cats captured around Seoul (including some boundary areas of Gyeonggi Province) from June to August 2013 through Hello Earth Co., an animal welfare and education consulting company in Korea. The feces of cats were stored at 4˚C until analyzed.
Nested-PCR for T. gondii B1 gene
The fecal samples (n=300) were examined for the presence of T. gondii B1 gene using nested-PCR. The DNeasy blood and tissue kit (Qiagen, Hilden, Germany) was used for isolation of the genomic DNA of T. gondii. Two primer sets directed against the B1 gene were used according to the previous study [14]. The smart 2× PCR Pre-mix (Solgent Co., Daejeon, Korea) was used with the following conditions: 93˚C for 5 min, 40 cycles of a denaturation step of 93˚C for 10 sec, annealing at 57˚C for 10 sec, and extension at 72˚C for 30 sec for first-round amplification. Nested reactions containing the first-round product were cycled 40 times with the following conditions: 93˚C for 10 sec, 62.5˚C for 10 sec, and 72˚C for 15 sec [14]. The PCR products were analyzed by 2% agarose gel electrophoresis and stained with ethidium bromide.
Fecal examination of oocysts
To confirm the presence of T. gondii oocysts in the B1 genepositive fecal samples, the sucrose flotation method was applied to concentrate the oocysts. Briefly, 2 g of feces were mixed with 10 ml sucrose solution (Sigma-Aldrich, St. Louis, Missouri, USA) with a specific gravity of 1.2, and filtered through a strainer into a 15-ml conical tube. The filtrate was then centrifuged at 1,200 rpm for 5 min at 4˚C. The tube was removed from the centrifuge and completely filled with sucrose solution and a coverslip was then placed on the tube. After 10 min, oocysts of T. gondii were identified on the coverslip under a light microscope at 1,000×magnification.
Bioassay of mice
Fourteen mice were orally inoculated each with 0.2 ml of fecal suspension from 14 T. gondii B1 gene positive cats. The brain tissue samples were obtained from each mouse 40 days after the inoculation. A portion of the brain tissue was squashed between a cover slip and a glass slide for microscopic detection of T. gondii tissue cysts.
Genotype analysis of 2 T. gondii isolates by PCR-nucleotide sequencing
PCR and nucleotide sequencing were performed on the SAG5D and SAG5E regions of T. gondii according to the procedures previously reported [15]. SAG5D (present in tachyzoites and encodes a polypeptide of 362 amino acids) and SAG5E (transcribed pseudogene) are novel SAG1-related genes useful for discrimination of virulent and avirulent strains of T. gondii [15]. The PCR product was amplified using the Cosmo Labopass ×2 PCR Premix kit (Cosmo Genetech, Seoul, Korea) with the following conditions: denaturation at 94˚C for 3 min, then 35 cycles at 95˚C for 20 sec, 56˚C for 30 sec, 72˚C for 80 sec, and post-amplification at 72˚C for 7 min. Automated DNA sequencing of the PCR product was performed by Solgent Co. (Seoul, Korea). Nucleotide sequences obtained from each of the 2 isolates were aligned using the Geneious v. 6.0.3. program (Geneious Co., Wellington, New Zealand).
Statistical analysis
Differences in the prevalence of T. gondii infection related to gender and age were statistically evaluated using the chi-square test in Excel. The differences were considered statistically significant when P<0.05.
RESULTS
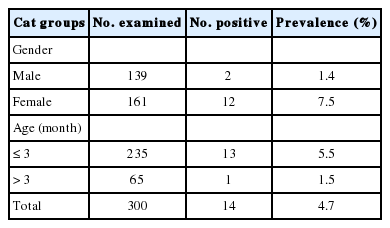
Fourteen (4.7%) of the 300 fecal samples of stray cats were positive for T. gondii B1 gene by nested-PCR (Table 1). Fecal oocysts were confirmed in at least 10 of the 14 positive feces (data not shown). Female cats (7.5%, 12/161) showed a higher B1 gene prevalence than male cats (1.4%, 2/139). By age, cats 3 months or younger (5.5%, 13/235) showed a higher prevalence than cats older than 3 months (1.5%, 1/65). These gender- and age-related differences were statistically significant (P<0.05).
Fecal samples positive for B1 gene were orally inoculated into 14 experimental mice, each with 1 fecal sample. After 40 days, 2 mice were positive for brain tissue cysts (Fig. 1). Thereafter, these 2 isolates have been continuously maintained in our laboratory. They were designated as KNIH-1 and KNIH-2 isolates.
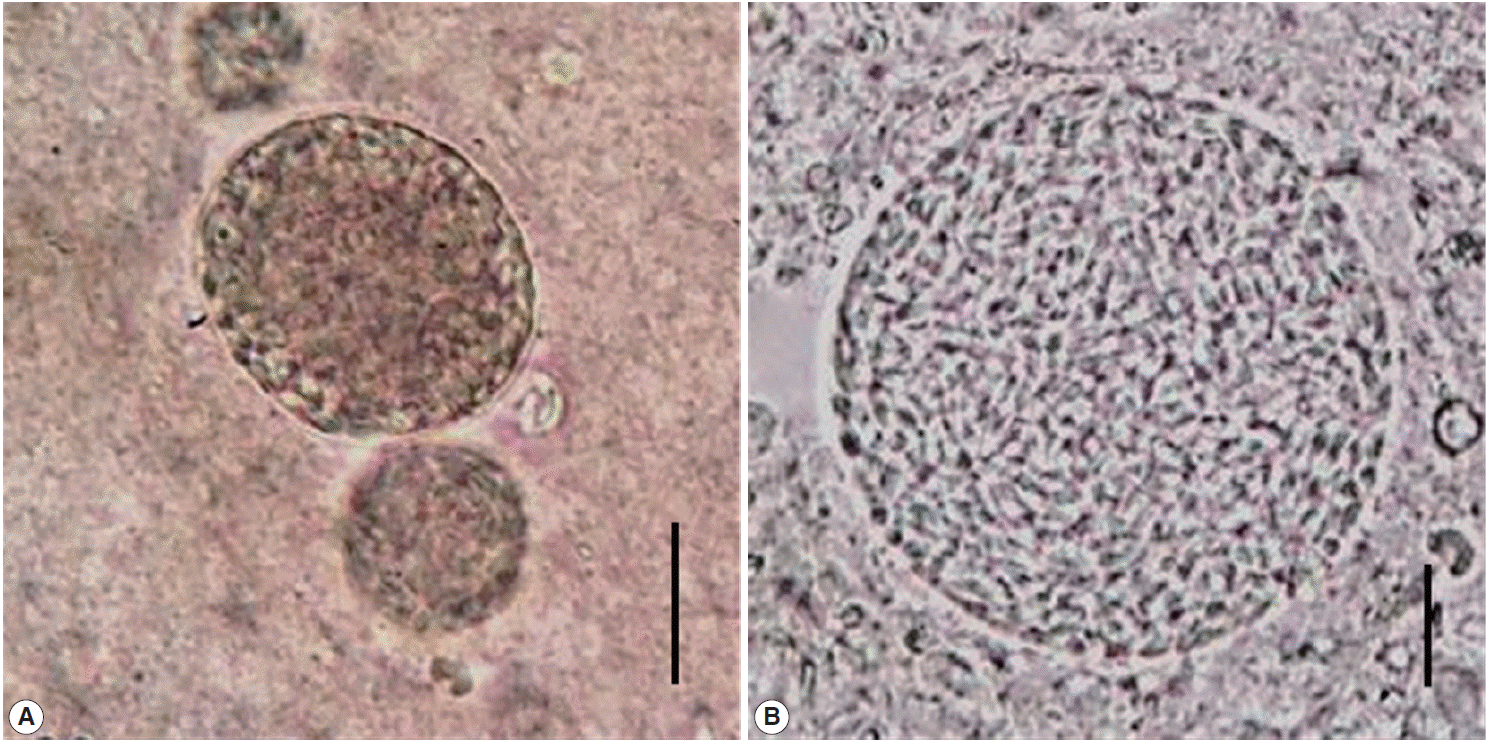
Tissue cysts in the brain of mice infected with T. gondii KNIH-1 (A) and KNIH-2 isolates (B) successfully passaged in this study. These 2 isolates showed 100% homology with the known ME49 strain based on SAG5D and SAG5E gene sequences (Scale bars=20 μm).
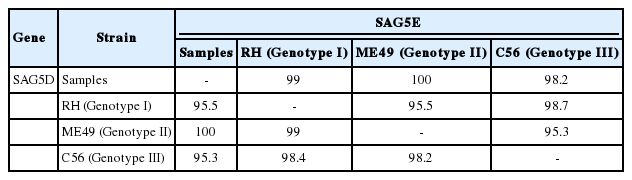
Genotyping of KNIH-1 and KNIH-2 isolates included a comparison of their SAG5D and SAG5E gene sequences with those of other strains deposited in GenBank, including RH (genotype I and highly virulent strain), ME49 (genotype II and less virulent strain), and C56 (genotype III and avirulent strain) (Table 2). The SAG5D sequences were identical between the 2 isolates (KNIH-1 and KNIH-2). The SAG5D sequences of the KNIH-1 and KNIH-2 isolates had 100% homology with the T. gondii ME49 strain (GenBank accession no. AY190528). However, the 2 isolates showed 99.0% identity with the RH strain (no. AY299523) and 98.2% identity with the C56 strain (no. AY190530). Similarly, the SAG5E sequences of KNIH-1 and KNIH-2 were identical to each other. They showed 100% homology with the ME49 strain (no. AY363047), whereas their homologies with RH (no. AY363043) and C56 strains (AY363049) were 95.5% and 95.3%, respectively (Table 2). These results indicated that the 2 laboratory-passaged isolates are highly homologous with a less virulent ME49 strain rather than a highly virulent RH or an avirulent C56 strain.
DISCUSSION
Several surveys have been reported on the seroprevalence or B1 gene detection of T. gondii infection in stray cats in Korea [3-5,7,8]. In 1999, 13.1% (26/198) of stray cats captured from a southern area (around Jinju City) showed positive western blot patterns for T. gondii [3]. In 2008, of 174 stray cats captured from Gyeonggi Province, serum specific antibodies were positive in 14 cats (8.0%) by latex agglutination test, 28 cats (16.1%) by ELISA, and 23 cats (13.2%) by PCR detection of the B1 gene in blood samples [4]. However, in the same year, a strikingly high prevalence (47.2%) in blood of stray cats by nested-PCR was reported from Gyeonggi Province [5]. Subsequently, the seroprevalence of stray cats from Metropolitan Seoul was reported as 15.3% (11/72) by ELISA, with the B1 gene positive rate in blood of 30.6% (22/72) by nested PCR in 2010 [7]. In another survey, the seroprevalence of stray cats from Metropolitan Seoul was 15.8% (69/456) by ELISA, and the B1 gene positive rate in blood was 17.5% (80/456) in 2011 [8]. These data demonstrate that the seropositivity and B1 gene (in blood) positivity of T. gondii infection in stray cats in Korea are markedly variable, ranging from 8.0-47.2%. The reasons for this wide range of reported prevalence include different areas surveyed, different analytical methods, and different materials (blood or feces) used for analyses. However, prevalence surveys using fecal samples were seldom performed in Korea.
In our study, the positive rate of B1 gene was 4.3% (14/300) using fecal samples (but not blood), which was much lower than the seroprevalence or B1 gene prevalence reported in blood samples [3,4,8]. Although the positive rate in this study was comparatively lower than others, we could confirm that fecal samples can be used for molecular studies including B1 gene detection. The lower positivity of T. gondii DNA in fecal samples than in blood is interesting. The reason may be that only the oocyst stage is excreted and available in feces, whereas various other T. gondii stages can be present in blood. In addition, cats shed oocysts in feces only for a short period of time (a few months) after infection, although the infection can continue for a substantially longer period of time and T. gondii specific IgG antibodies in serum persist in cats [16]. Therefore, surveys to detect fecal oocysts in cats are less reliable than detection of B1 gene in blood or antibodies in sera, to understand the true prevalence of T. gondii infection. The true prevalence of T. gondii in stray cats in Korea seems to be at least higher than 10% and can reach 50% according to the literature.
The prevalence of T. gondii in cats may depend on the locality, living conditions, as well as age and gender of the cats. However, in Korea, there have been few surveys on cats from different localities. It is speculated that cats living in rural areas may have a higher prevalence of T. gondii than those living in cities including Seoul. Furthermore, stray cats that hunt for food have a higher prevalence of T. gondii than those domesticated and fed with preserved food. In Korea, household cats from Seoul revealed a remarkably lower T. gondii seroprevalence (2.2%, 10/437) and B1 gene prevalence (2.1%, 10/474) [6], as compared to stray cats with 8.0-17.5% and 13.2-47.2%, respectively [3-5,7,8]. Another survey of household cats reported 0% (0/40) seroprevalence by ELISA (15.3% in stray cats in the same study) and 0% B1 gene prevalence by nested PCR (30.6% in stray cats in the same study) [7].
In our study, the younger cat group (≤3 months) revealed a significantly higher prevalence of T. gondii (5.5%) than the older cat group (>3 months) (1.5%). This contrasted to the general statement that the seroprevalence of T. gondii in cats increases with ages [16,17]. It may be explained by that fecal oocysts are shed by infected cats only for a short period of time after exposure, and the infection can continue for a substantially longer period of time [16]. In our study, female cats (7.5%) revealed a significantly higher prevalence than male cats (1.4%). This gender difference was difficult to explain. However, no significant gender difference has previously been reported in the seroprevalence or B1 gene prevalence of T. gondii infection in Korea [3-8].
The study confirmed that stray cats play an important role for transmission of T. gondii infection in Korea. Among the 14 B1 gene positive samples, 2 were successfully passaged in the laboratory using mice. They were genetically confirmed as 100% homologous with the ME49 strain (genotype II) in the sequences of SAG5D and SAG5E [15]. The results of the present study raise public health awareness and contribute to integrated strategies of health initiatives for prevention and control of T. gondii infection in Korea.
Acknowledgements
This study was supported by a research grant from The Korea National Institute of Health, Korea Centers for Disease Control and Prevention, Ministry of Health and Welfare, the Republic of Korea (no. 800-2013-E54007-00). We would like to thank the staff of Hello Earth for providing the fecal samples of stray cats for this study.
Notes
The authors declare that they have no competing interests.