Protective effect of lectin from Synadenium carinatum on Leishmania amazonensis infection in BALB/c mice
Article information
Abstract
The protective effect of the Synadenium carinatum latex lectin (ScLL), and the possibility of using it as an adjuvant in murine model of vaccination against American cutaneous leishmaniasis, were evaluated. BALB/c mice were immunized with the lectin ScLL (10, 50, 100 µg/animal) separately or in association with the soluble Leishmania amazonensis antigen (SLA). After a challenge infection with 106 promastigotes, the injury progression was monitored weekly by measuring the footpad swelling for 10 weeks. ScLL appeared to be capable of conferring partial protection to the animals, being most evident when ScLL was used in concentrations of 50 and 100 µg/animal. Also the parasite load in the interior of macrophages showed significant reduction (61.7%) when compared to the control group. With regard to the cellular response, ScLL 50 and 100 µg/animal stimulated the delayed-type hypersensitivity (DTH) reaction significantly (P < 0.05) higher than SLA or SLA plus ScLL 10 weeks after the challenge infection. The detection of high levels of IgG2a and the expression of mRNA cytokines, such as IFN-γ, IL-12, and TNF-α (Th1 profiles), corroborated the protective role of this lectin against cutaneous leishmaniasis. This is the first report of the ScLL effect on leishmaniasis and shows a promising role for ScLL to be explored in other experimental models for treatment of leishmaniasis.
INTRODUCTION
The increased incidence of the American cutaneous leishmaniasis (ACL) associated with drug resistance in patients with acquired immunodeficiency syndrome (WHO, 1998) justifies the urgency of attaining new methods of control and prophylaxis for this intracellular infection. The solid immunity observed after ACL recovery in human beings has stimulated numerous studies for the development of a prophylactic vaccination (Machado-Pinto et al., 2002; Späth et al., 2004). Purified antigens, subcellular fractions and parasites of low virulence were used as potential candidates for a vaccine against cutaneous leishmaniasis (Cardoso et al., 2003). DNA vaccines being used are able to induce strong humoral and cellular immune responses, presenting the potential for increasing immunogenicity through vector modification or incorporation of cytokine genes as the adjuvant (Ivory and Chadee, 2004; Marques-da-Silva et al., 2005). T cells mediate the acquired resistance in leishmaniasis. This idea has been supported by classical experiments establishing that mice deficient in T lymphocytes quickly die after parasite inoculation, and also that the transference of normal T lymphocytes confers resistance to these animals (Scott et al., 1988).
In murine models of infection with Leishmania major, susceptive mice generally promote higher Th2 response than Th1 response (Alexander and Bryson, 2005). However, in mice infected with Leishmania donovani, the distinguishing production of Th1 and Th2 cytokines does not control the cure; even though the Th1 response relates to resistance to the infection, the Th2 response does not determine susceptibility (Kaye et al., 1991). Moreover, the lack of a powerful adjuvant with in vivo activity dramatically limits the development of effective formularizations of vaccines. The ideal adjuvant for a vaccine against leishmaniasis that induces lasting protection will have to attract and to activate the response of antigen-presenting cells (APCs) directing a differentiation of the Th1 type cellular response (Moingeon et al., 2001). Different compounds have been evaluated with respect to the adjuvant activity and security. Hydroxide and phosphate of aluminum remain the only compounds licensed for use in human beings.
The practice of herbal or naturalistic medicine has each time more followers. That is due, in part, to the recognition of the value of traditional therapies, particularly of Asian origin, and the identification of medicinal plants derived from the aboriginal pharmacopeias, which present significant power of cure and are found in abundance in tropical countries (Elvin-Lewis, 2001). In Brazil, about 80,000 vegetal species with therapeutic properties have been described, and amongst new compounds studied are saponins and lectins.
The lectins are involved in initial stages of many molecular biological interactions, such as the intracellular translocation of glycoproteins and soluble molecules, in addition to involvement in the regulation of endocytosis, cell migration, adhesion, and immune defense processes. The capacity of the lectins to interact specifically with carbohydrates makes them valuable instruments in varied biological research work, and the use of these tools has expanded continuously (Peumans and van Damme, 1995).
The lectins are widely distributed in nature in seeds, leaves, barks, bulbs, rhizomes, roots, cotyledon and tubers depending on the plant species. Lectins, such as the ConBr from Canavalia braziliensis and KM+ from Artocarpus integrifolia induce IFN-γ and IL-12 p40 production and promote an inversion of the Th2 to Th1 cytokine pattern in BALB/c mice infected with L. amazonensis and L. major, respectively (Barral-Netto et al., 1996; Panunto-Castelo et al., 2001). Saponins, such as from Quillaja spp, Quillaja saponaria, and the conformational isomers of QuilA sapogenin, have been shown to increase the cutaneous response to Leishmania antigens and enhance serum levels of IFN-γ, and may protect against murine infections with L. major, L. donovani and Leishmania chagasi (Souza et al., 2004; Palatnik de Sousa et al., 2004; Oliveira-Freitas et al., 2006).
Synadenium carinatum is a common plant in Brazil, and an aqueous preparation from its latex has been widely and indiscriminately used in popular medicine to treat a great number of inflammatory disorders. Recently, we isolated, purified, and characterized a D-galactose-binding lectin that was extracted from the latex, and this plant was named ScLL (Souza et al., 2005). In the present study, we investigated the effect of immunization with lectin from S. carinatum on L. amazonensis infection in BALB/c mice by examining Th1 immune responses, such as delayed-type hypersensitivity (DTH) reaction, cytokines, and IgG isotypes, involved in protection against murine leishmaniasis in a Th2 susceptible mouse model.
MATERIALS AND METHODS
Preparation of the latex extract and purification of the lectin
Popularly known as "Leiteirinha or Folha Santa", the S. carinatum species was harvested in Uberlândia, Minas Gerais, and registered in the Herbarium of the Universidade Federal de Uberlândia (HUFU 38354) (Souza et al., 2005). The proteins were extracted after homogenization of the S. carinatum latex with deionized water in the ratio of 1: 5 at 4℃ for 48 hr. The mixture was centrifuged at 3,500 g for 30 min, at 4℃, filtered in a nitrocellulose membrane (0.45 µm; Merck, Göttingen, Germany), originating the crude extract. The D-galactose-binding lectin (ScLL) was purified on immobilized D-galactose-agarose column (Pierce, Rockford, Illinois, USA), balanced with 0.05 M borate-buffered saline (BBS), pH 7.2. The ScLL was eluded with 0.4 M D-galactose in BBS (BBS-D-gal) and dialyzed against Tris buffer (pH 7.2). The protein concentration was determined (Lowry et al., 1951), and the ScLL was stored at -20℃ until use.
Parasites and preparation of the soluble Leishmania antigen (SLA)
Leishmania amazonensis (IFLA/BR/67/PH8) promastigote forms obtained from a primary culture were kept in a brain heart infusion (BHI) medium (Oxoid, Basingstoke, UK) supplemented with 10% of fetal bovine serum (Cultilab, Campinas, Brazil) at 28℃. The preparation of SLA was carried out in accordance with Scott et al. (1987). The parasites were harvested from the culture medium and washed 4 times by centrifugation at 4℃, for 15 min at 3,000 g in a sterile phosphate-buffered saline solution (PBS), pH 7.2. The concentration of parasites was adjusted to 1 × 109/ml in PBS containing 50 µg/ml leupeptin and 1.6 mM phenylmethylsufonyl fluoride (Sigma, St. Louis, Missouri, USA) and incubated in ice-water bath for 10 min. The parasites were then lysed by freeze-thaw and sonication in an ice bath. The lysate was centrifuged at 3,000 g for 30 min at 4℃ and the supernatant was centrifuged again at 10,000 g for 30 min at 4℃. The supernatant was harvested, filtered through a 0.22 µm membrane and the protein concentration determined and then stored at -70℃ until use.
Immunization and infection of mice
Isogenic BALB/c mice, 6-8 week-old, were used in the experiments. Groups of 5 animals were inoculated by the subcutaneous route with PBS (control group, 50 µl/animal), SLA (25 µg/animal), SLA (25 µg/animal) plus ScLL (10, 50, 100 µg/animal), and only ScLL (10, 50, 100 µg/animal) in the right footpad. The animals received 3 immunization doses with intervals of 15 days. Three days after the last immunization, the animals were challenged in the left hind footpad with 1 × 106 stationary phase promastigotes of L. amazonensis. The evolution of the infection was monitored weekly through the volume measurement of footpad swelling using a manual caliper (Mitutoyo, Tokyo, Japan), during 10 weeks. The measurements between the infected footpad and the contralateral uninfected footpad refer to differences in the lesion size, in millimeters.
Induction and measurement of DTH
To evaluate the cellular immune response, the animals of each group were submitted to the DTH reaction at week 10 after the challenge. The infected footpad and the uninfected contralateral footpad were measured using a manual caliper before the inoculation of the SLA antigen. The antigen was inoculated in the footpad of the animals in the concentration of 50 µg/animal and evaluated after 48 hr. The difference between the 2 measures was expressed in millimeters. The test was considered positive when the value of the difference between the 2 measures was greater than or equal to 0.05 mm (Barral-Netto et al., 1983).
Serologic analysis
Levels of the total serum immunoglobulin G (IgG) and their subclasses (IgG1 and IgG2a) were determined by ELISA. The 96-well plates (Montegrotto, Padova, Italy) were coated with SLA of L. amazonensis (10 µg/ml) in the carbonate buffer (pH 9.6), during 18 hr at 4℃. Afterwards, the plates were washed with PBS containing 0.05% Tween 20 (PBST), serum samples of mice diluted 1: 100 were added and then incubated at 37℃ for 45 min. The plates were washed again with PBST and the secondary antibody (peroxidase-conjugated anti-isotype murine rat IgG) (Zymed Laboratories Inc, San Francisco, California, USA) was added and incubated at 37℃ for 45 min. The plates were washed again and the enzymatic substrate (perhydrol in phosphate/citric acid buffer pH 5.0 containing orthophenylenediamine, Sigma) was added. After 15 min of color development, the reaction was stopped with 2N H2SO4 and the antibody levels were determined by reading the absorbance at 492 nm (Titertek Multiskan Plus, Flow Laboratories, Switzerland).
Histopathology
BALB/c mice of all the immunized and control groups suffered euthanasia in accordance with the Brazilian College of Animal Experimentation, 10 weeks after challenge with L. amazonensis. Fragments of the footpad and popliteal lymph nodes were removed, fixed in 10% formalin solution and dehydrated in successive alcohol concentrations (50-100%). After dehydration, the tissues were absorbed, enclosed in paraffin and sectioned by microtome (Leica Instruments Gmbh, Nussloch, Germany). Cuts of 4-6 µm of thickness were stained with hematoxylin and eosin (H-E) and analyzed by light microscopy. The evaluation of parasite burden in footpads of infected mice was performed by counting the amastigotes inside macrophages. Microscopic images were transmitted through an Olympus 200 screen connected to a microscope BX 40 (Olympus Optical Co. Ltd., Tokyo, Japan) and analyzed according to software HLImage++97 (Western Vision Software, East Layton, Utah, USA). The images were analyzed in 97,000 mm2 (30 fields) per group.
Cytokines and inducible nitric oxide synthase (iNOS) expression by conventional RT-PCR
RNA was isolated from the lesion in the footpad of the immunized mice and controls 10 weeks after infection with L. amazonensis, using the RNA kit (RNeasy Mini Kit, Qiagen, Valencia, California, USA) according to the manufacturer's instructions. The cDNA was obtained using an iScript™ cDNA synthesis kit (Bio-Rad Laboratories, Hercules, California, USA). The PCR reaction used 8 µl of master mix (0.2 mM dNTP, 1 µl 10x PCR buffer, 0.2 µM of each INF-γ, IL-12, IL-4, TNF-α, and iNOS primer), 4.4 µl water, 1 U/µl Taq DNA polimerase, 1.5 mM MgCl2 (Invitrogen Life Technologies, Carlsbad, California, USA) and 2 µl cDNA of each sample. The sequence of the primer used is listed in Table 1. The reaction was carried out in a thermocycler (Applied Biosystems, Foster City, California, USA) with the following program: 95℃/2 min, 94℃/1 min, 57℃/1 min, and 72℃/1 min, for 37 cycles. When the PCR products finished, 8 µl were added to 2 µl of buffer sample and submitted to 2% agarose gel electrophoresis containing 1 µg/ml ethidium bromide. It was glycerilaldehyde-3-phosphate dehydrogenase (GAPDH) primer as the normal pattern of expression. The bands were visualized under ultraviolet light (Höefer Pharmacia Biotech, San Francisco, California, USA) and photographed (Polaroide FisherBiotech, Cambridge, Massachusetts, USA). The efficiency-corrected quantification performed automatically by relative expression (RE) software was based on relative standard curves describing the PCR efficiencies of the used cytokines and GAPDH genes. The values were within the linear range of standard curve, and results were expressed as the cytokine/GAPDH ratio (the test sample divided by cytokine/GAPDH ratio of the calibrator sample × 100%), and therefore, were corrected for samples in homogeneity and detection-caused variances.
Statistical analysis
Statistical analysis was carried out using two-tailed Student's t-test for unpaired samples. The differences that provided P < 0.05 were considered statistically significant. The software GraphPad Prism 4 for Windows (GraphPad Software, Inc., San Diego, California, USA) was used. All experiments were carried out in triplicate.
RESULTS
Lesion progression and parasite burden
The immunization with SLA associated to ScLL conferred partial protections for the animals (Fig. 1A) with statistical significance (P < 0.05). When ScLL alone was used in the concentration of 100 µg/animal, the protection that was conferred to the animals was higher compared to the SLA plus ScLL (Fig. 1B; P < 0.05). Despite the fact that the group immunized with SLA associated to ScLL (100 µg/animal) had conferred early protection in the animals in the 6th week after the infection (Fig. 1A; P < 0.0001). BALB/c mice immunized with ScLL only (10, 50 or 100 µg/animal) were shown to be partially protected at the end of 10 weeks, when compared with the groups immunized with SLA only and control (Fig. 1B). The differences in the lesion progression between these groups were statistically significant from the 8th week after infection (P < 0.05). The parasite burden in the footpad of the animals immunized with SLA and/or ScLL was evaluated after 10 weeks of infection by L. amazonensis by counting the amastigote forms present in macrophages. The analysis of the captured images disclosed that the groups of animals immunized with ScLL (10, 50 or 100 µg/animal) presented statistically significant reduction in parasite burdens when compared with the groups immunized only with SLA and control (Fig. 1C; P < 0.0001). The group immunized with 100 µg/animal of ScLL presented 61.7% of reduction in the parasite burden when compared to the control, while in the animals immunized with 10 and 50 µg/animal of ScLL there was reduced intracellular parasitism (45.1% and 57.4%, respectively) (Fig. 1C). In a similar way, statistically significant reduction in the parasitism was observed when the association with SLA and ScLL (10, 50 or 100 µg/animal) was used (Fig. 1C; P < 0.05) compared to the group of mice immunized only with SLA.
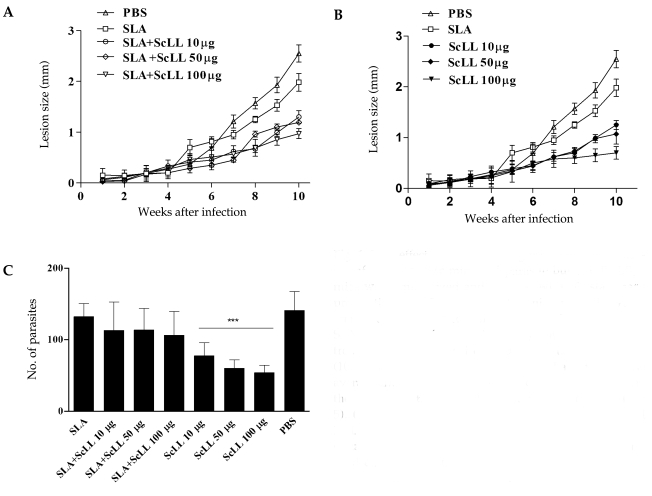
ScLL effect in the lesion progression of L. amazonensis-infected BALB/c mice and parasite burden. BALB/c mice were immunized and infected with 106 stationary promastigotes of Leishmania and monitored for 10 weeks after infection. (A) Mice immunized with 25 µg/animal of SLA, SLA plus ScLL (10, 50 or 100 µg/animal), and control. (B) Mice immunized with 25 µg/animal of SLA, ScLL (10, 50 or 100 µg/animal), and control. Bars indicate the average and the standard deviation of differences between the average of the infected and uninfected footpads (n = 5). (C) Parasite burden in the footpad of immunized BALB/c mice with SLA and/or ScLL and infected by L. amazonensis. The results express the average and the standard deviation of the number of parasites by field into infected macrophages. ***P < 0.0001.
DTH
After 10 weeks of infection, the cell-mediated immunity was determined by the measurement of DTH. BALB/c mice developed changeable levels of DTH. The immunizations with SLA plus ScLL (10, 50 or 100 µg/animal) showed negative DTH responses (Fig. 2). Only 40%, 80%, and 60% of the animals immunized with ScLL (10, 50 and 100 µg/animal, respectively) showed DTH responses equal or upper that 0.05 mm being considered positive (Fig. 2).
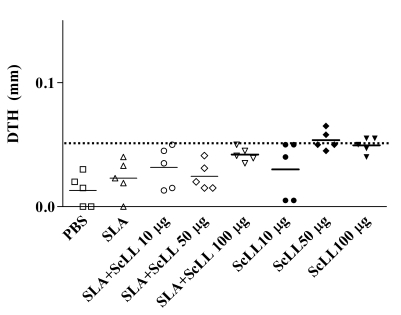
DTH measurements. At the 10th week after infection with L. amazonensis, the immunized mice received 50 µg SLA antigen on the uninfected contralateral footpad. After 48 hr, the reading of the skin endurance was carried out as described in Materials and Methods. SLA (25 µg/animal) alone, SLA (25 µg/animal) plus ScLL (10, 50 or 100 µg/animal), PBS (control), and ScLL (10, 50 or 100 µg/animal) group mice. Bars indicate median for the measurement of the skin endurance in each group (n = 5). The line indicates 0.05 mm.
Humoral immune responses
All the immunized animals developed specific anti-L. amazonensis IgG antibodies. The levels of the total IgG of the groups immunized with SLA plus 100 µg/animal ScLL and ScLL alone (10, 50 and 100 µg/ animal) were significantly lower than the control group (Fig. 3A; P < 0.05). The serum sample of animals immunized with 100 µg/animal ScLL plus SLA and ScLL alone (10, 50 and 100 µg/animal) showed low levels of IgG1 when compared to the groups immunized with SLA or control (Fig. 3B; P < 0.05). The levels of anti-Leishmania IgG2a antibodies were significantly higher in serum samples from the groups of mice immunized with ScLL alone (10, 50 and 100 µg/animal) than in serum samples from the controls (Fig. 3C; P < 0.01).
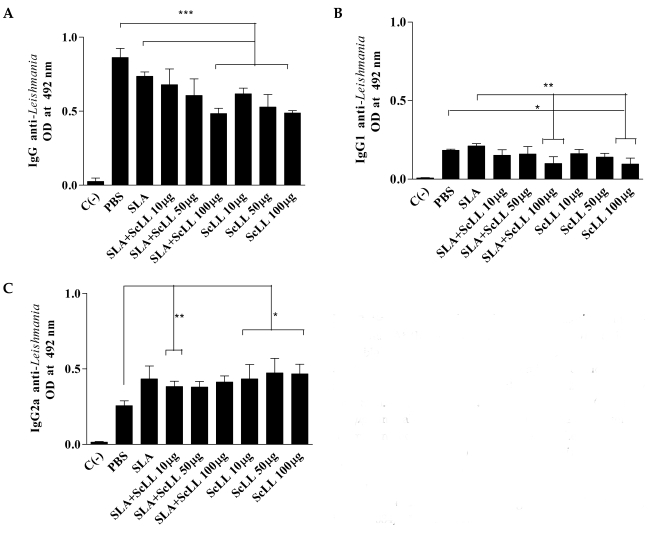
Serum levels of specific anti-Leishmania immunoglobulins. At the 10th week after infection, the animals were bled and evaluated according to the level of specific antibodies by ELISA. Control (PBS) mice, and mice immunized with SLA (25 µg/animal) alone, SLA (25 µg/animal) plus ScLL (10, 50 or 100 µg/animal), and ScLL (10, 50 or 100 µg/animal) were analyzed. C (-) represents the sera of unimmunized and uninfected mice. (A) Levels of anti-Leishmania total IgG. (B) Levels of anti-Leishmania IgG1. (C) Levels of anti-Leishmania IgG2a. Bars express the average and the standard deviation of optical density (OD) at 492 nm in serum samples of each group (n = 5). *P < 0.05; **P < 0.0084; ***P < 0.0001.
Histopathology
After 10 weeks of infection with L. amazonensis, histopathological alterations varying in intensity were evidenced in all the immunized mice and controls (Figs. 4A-4H). The cells of dermis, epidermis, and hypodermis in the infected footpad had shown intense parasitism and thickening of keratin that recovers the epidermic extract (hyperkeratosis). The main alterations were found in dermis and hypodermis beyond typical alterations of a chronic granulomatous inflammatory process, in the lesion site as well as in the adjacent popliteal lymph node (Figs. 4A and 4B, respectively). The main histopathological findings were hyperplasia and hypertrophy of the cells present in the dermis, especially macrophages and fibroblasts (Figs. 4E and 4G). Focal areas of necrosis were observed in the skin and in popliteal lymph nodes (Fig. 4A and 4B, respectively). Cellular infiltrate composed by lymphocytes, plasma cells, neutrophils, and eosinophils were also observed (Figs. 4A-4H). The macrophages present in the footpad lesion and popliteal lymph node draining showed large vacuoles heavily parasitized and the cytoplasm and nuclei dislocated to the periphery of the cell (Fig. 4A and 4B, respectively). Therefore, the higher parasite density in macrophages was observed in the footpad lesion and in the popliteal lymph node of the control animals (Fig. 4A and 4B, respectively) and SLA group animals (Fig. 4C and 4D, respectively). The immunized ScLL group (100 µg/animal) showed diminished parasitism of macrophages in footpad lesion (Fig. 4G) and predominantly in the lymph node (Fig. 4H) when compared to the control group.
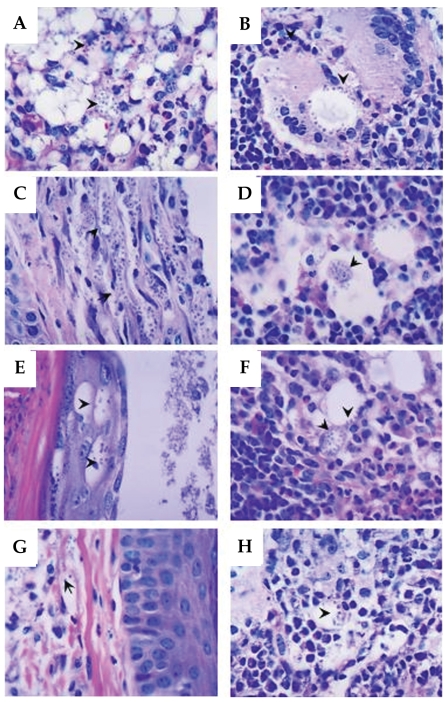
Histopathological features. (A) PBS group (skin) showing mononuclear exudate, neutrophils in the dermis and hypodermis, and parasitism of macrophages (arrowheads). (B) PBS group (popliteal lymph node) showing multinuclear giant cells with parasites, vacuoles with numerous amastigote forms in the periphery (arrowheads). (C) SLA group (skin) parasitism of the elongated cells of dermis compatible with fibroblasts (arrowheads). (D) SLA group (popliteal lymph node) parasitism of macrophages (arrowheads). (E) SLA + 100 µg/animal ScLL group (skin) parasitism of epidermal keratinocytes (arrowheads). (F) SLA + 100 µg/animal ScLL group (popliteal lymph node) eosinophilic exudate, and parasitism of macrophages (arrowheads). (G) 100 µg/animal ScLL group (skin) parasite-containing macrophages (arrowheads) and dermal fibroblasts. (H) 100 µg/animal ScLL group (popliteal lymph node) parasitism of macrophages (arrowheads). Data are representative of all immunized and infected mice with L. amazonensis. H-E stain. Original magnifications × 1,000.
Expression of cytokines and iNOS
After 10 weeks of infection with L. amazonensis, it was observed by conventional RT-PCR that the different concentrations of ScLL used in the immunization schedule induced the expression of different cytokines and iNOS in the lesion site. INF-γ was expressed in all group mice, but none was detected in the SLA group (Fig. 5). IL-12 expression occurred with higher intensity in the groups immunized with ScLL (50 and 100 µg/animal; Fig. 5). TNF-α was expressed with higher intensity in the groups immunized with SLA alone, SLA plus ScLL (50 µg/animal), and ScLL alone (50 µg/animal). However, it was shown in lesser intensity in the group of mice immunized with 10 and 100 µg/animal ScLL alone (Fig. 5). IL-4 expression was evidenced in all immunized groups, including the control group, although with lower intensity in the ScLL groups (10 and 100 µg/animal; Fig. 5). The iNOS enzyme was not detected in the control group, but was expressed in all other groups, with a higher intensity in the ScLL groups (10 and 100 µg/animal) (Fig. 5).
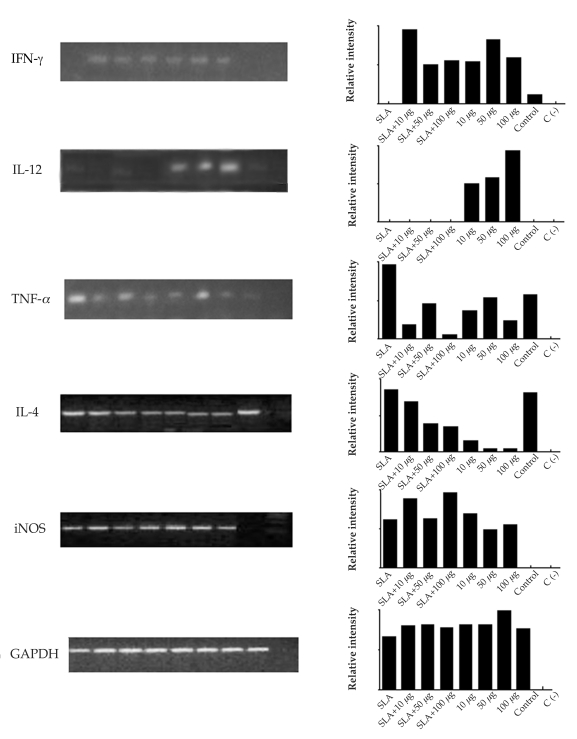
Expression and relative intensity of cytokines and iNOS by conventional RT-PCR assay in the lesion footpads of immunized mice and mice challenged with L. amazonensis. GAPDH expression was used as normal patterns of mRNA expression. Bars represent the relative intensity of the cytokines and iNOS expression. SLA corresponded to immunizing mice with SLA alone; SLA+10 µg, SLA+50 µg, SLA+100 µg corresponded to immunizing mice with SLA+ScLL 10 µg/animal, SLA+ScLL 50 µg/animal, SLA+ScLL 100 µg/animal, respectively; 10 µg, 50 µg, 100 µg, corresponded to immunizing mice with ScLL 10 µg/animal, ScLL 50 µg/animal, ScLL 100 µg/animal, respectively; and control corresponded to immunizing mice with PBS. C (-): negative reaction control.
DISCUSSION
In this work we evaluated the effect and a possible adjuvant role of the S. carinatum lectin (ScLL) in the immunization of BALB/c mice associated, or not, with L. amazonensis soluble antigen (SLA). Different concentrations of ScLL were used in this study due to the absence of previous standardization, since no study using this lectin was carried out until now. The immunization of BALB/c mice with different concentrations of ScLL associated, or not, with SLA resulted in reduced lesions. The immunization only with SLA resulted in lesions almost as evident as in the controls immunized only with PBS. Similar results had been described previously (Liew et al., 1985; Barral-Netto et al., 1987). There was evidence that, while the endovenous or intraperitoneal immunization with non-living promastigotes forms killed by heat or irradiation, promotes a lasting protection, the intramuscular and subcutaneous immunization with the same antigens increase the susceptibility, aggravating the lesion. This occurs in disease-resistant genetically modified animals (CBA mice) and in susceptible animals (BALB/c mice). The intravenous immunization of BALB/c mice, with soluble antigen of promastigote forms of L. amazonensis resulted in a partial protection of them. These animals started to present histopathological features with the presence of granulomatous infiltrations, deposit of collagen and fibrin necrosis, and lesions similar to those observed in resistant mice (Andrade et al., 1984).
In the present study, mice immunized with ScLL presented smaller lesions in the footpads during the 10 weeks of observation. This was shown by histopathological analysis demonstrating the presence of granulomatous cellular infiltrations, deposit of collagens, necrosis, and significant reduction of the local parasite load in relation to the control animals. These results suggest that ScLL was capable of partially protecting the animals, conferring them the lesions characteristic of animals that are controlling the infection, which is corroborated by the reduced intracellular parasitism. Similar results were reported in immunized BALB/c mice with KM+ lectin of Artocarpus integrifolia, associated, or not, with SLA, and partial protection of the animals was observed after challenge with L. amazonensis (Teixeira et al., 2006).
The DTH is a reaction that characterizes the development of a specific cellular response, indicating a previous exposure to the antigen. In the present study, only groups of animals immunized with ScLL presented results acceptable as a positive DTH. Beyond the DTH, other factors, such as reduction of the lesion size, reduction of the parasite load, cytokine expression related to Th1 profile, and production of specific antibodies against the parasite, also suggest protection. However, the role of antibodies in cutaneous leishmaniasis is not clear yet. What is generally accepted is that the protective response against this illness is essentially cellular and antibodies do not present a determinative role (Liew and O'Donnell, 1993).
In leishmaniasis, resistance and resolution of the illness have been associated with IgG2a production (Mohammadi et al., 2006) and activation of Th1 cells with secretion of IL-2 and IFN-γ. In mice, IL-4 secreted by Th2 cells induces an immunoglobulin isotype switch on B lymphocytes to IgG1 and IgE, whereas IFN-γ produced by Th1 clones increased the response of the IgG2a isotype (Stavnezer, 1996). In a L. infantum model, it was shown that the levels of IgG1 antibodies and the specific anti-Leishmania IgG2 subclass detected in the dog sera could be considered as a predictor of progression and cure of the infection, respectively (Deplazes et al., 1995). Still, according to these authors, high levels of specific anti-Leishmania IgG antibody would be related to dissemination of the parasite. In a recent report, higher levels of anti-Leishmania IgG2a antibodies in sera from IL-4 deficient mice were associated with resistance to the infection (Guimarães et al., 2006). In the present study, the serological data is in accordance with these authors. The low levels of specific anti-L. amazonensis IgG antibody detected in sera of the animals immunized with different concentrations of ScLL suggests that the illness in mice immunized with ScLL is being controlled, while in the control animals the infection develops or progresses. Moreover, high levels of anti-L. amazonensis IgG2a were detected in serum samples of animals immunized with the ScLL lectin, suggesting a partial protection or control of the infection in these animals. Still, the low levels of IgG1 antibodies detected in sera of immunized mice support the idea that infection by Leishmania is being controlled in these animals.
In the present study, IL-12 and IFN-γ were expressed in the lesion, which is in agreement with the findings of other authors who had shown that these 2 cytokines are important for the development of protective immune responses in experimental leishmaniasis (Panunto-Castelo et al., 2001; Hernández et al., 2006). The ample expression of iNOS by immunized animals suggests that there was production of nitric oxide (NO) in the lesion, leading us to believe that the NO, together with IL-12 and TNF-α, acts in an important and synergistic way in mediation of the local inflammatory process. In the present study, the use of ScLL lectin (all concentrations) was capable of diminishing the lesion size developed and reducing significantly the parasite load in macrophages. Such a protective effect by immunization with ScLL alone appears to be due to a potent anti-inflammatory activity. In this way, there was inhibition of leukocyte accumulation in murine models of acute and chronic asthma inflammation after ScLL administration (Rogerio et al., 2007). However, this is the first report of the ScLL effect on leishmaniasis infection and has shown a promising role of ScLL in this immunization model, although the mechanism responsible for this protection is still unclear.
ACKNOWLEDGMENTS
We thank Dr. Maria I. Machado (UNIMINAS) for many suggestions, Dr. Henrique C. Teixeira (UFJF) for correction of the manuscript, and Dr. Cristina R. Barros Cardoso (USP) for the IgG detection assay.
References
Notes
This work had a financial support from Fundação de Amparo à Pesquisa de Minas Gerais (FAPEMIG), Brazil, and Sustainable Sciences Institute (SSI), California, USA.
