Effect of Diclazuril on the Bursa of Fabricius Morphology and SIgA Expression in Chickens Infected with Eimeria tenella
Article information
Abstract
The effects of diclazuril on the bursa of Fabricius (BF) structure and secretory IgA (SIgA) expression in chickens infected with Eimeria tenella were examined. The morphology of the BF was observed by hematoxylin and eosin staining, while ultrastructural changes were monitored by transmission electron microscopy. E. tenella infection caused the BF cell volumes to decrease, irregularly arranged, as well as, enlargement of the intercellular space. Diclazuril treatment alleviated the physical signs of damages associated with E. tenella infection. The SIgA expression in BF was analyzed by immunohistochemistry technique. The SIgA expression increased significantly by 350.4% (P<0.01) after E. tenella infection compared to the normal control group. With the treatment of diclazuril, the SIgA was relatively fewer in the cortex, and the expression level was significantly decreased by 46.7% (P<0.01) compared with the infected and untreated group. In conclusion, E. tenella infection in chickens induced obvious harmful changes in BF morphological structure and stimulated the expression of SIgA in the BF. Diclazuril treatment effectively alleviated the morphological changes. This result demonstrates a method to develop an immunological strategy in coccidiosis control.
INTRODUCTION
The lymphoid organs of chickens are divided into “central” and “peripheral”. The primary site for the development of lymphocytes is in the central lymphoid organ, i.e., the bursa of Fabricius (BF) [1]. The BF is unique peculiar to birds and dorsally located on the proctodeal portion of the cloaca. It is a single oval sac with a collum enclosing a luminal system which forms a slit-like opening through the collum into the cloaca [2]. The BF reaches its maximum size at 8-10 weeks of age and then gradually undergoes involution after the development of sexual maturity, and eventually vanishing.
The BF is responsible for the development and differentiation of B-lymphocytes. It has been shown that hematopoietic stem cells first enter the BF via the bloodstream, and then migrate into the mesenchyme towards the epithelium of the BF to form the lymphoid follicle. The development, maturation, and differentiation of B-cells in birds depend on the normal structure of the BF [3]. In an earlier report, it was demonstrated that surgical bursectomy or chemical removal of the BF profoundly disrupts the normal process of B-cell development, thereby seriously inhibiting the immune function of the BF [4].
Avian coccidiosis is recognized as an important disease in the field of poultry husbandry all over the world [5,6]. Infected birds display decreased feed intake leading to a reduction in weight gain, poor feed conversion, bloody diarrhea, which equates to high levels of morbidity and mortality rates [7]. Eimeria tenella is one of the most important Eimeria species, as it causes intestinal coccidiosis in chickens, imposing enormous economic losses.
Nowadays, coccidiosis is controlled mainly by anticoccidial drugs supplemented in the feed or water. Diclazuril, a benzeneacetonitrile broad-spectrum anticoccidial agent for broiler chickens, is highly efficacious against both the asexual and sexual stages of E. tenella [8,9]. Owing to the widespread use of these drugs in the poultry industry, the development of resistant pathogenic strains and drug residues in animal products have become major concerns [10]. Therefore, to explore an immunological strategy in coccidiosis control is extremely important [11-15].
The immune response to the parasites is complex, including specific and nonspecific immunity. Specific immunity is mediated by antibodies, lymphocytes, and cytokines. Immune responses mediated by antibodies and cells are both activated following infection. In response to challenge infection with Eimeria spp., chickens produce 3 classes of antibodies, IgY, IgA, and IgM [16]. IgA has 2 forms: serum IgA and secretory IgA (SIgA). SIgA is formed in 2 stages: B lymphocytes produce at least 2 monomeric IgA molecules, which dimerize covalently by the J-chain. IgA dimers (dIgAs) bind to the extracellular portion of the polymeric immunoglobulin receptor (pIgR), known as the secretory component. The oligomeric SIgA is taken up and transported across the epithelial cells and into secretions such as tears, saliva, sweat, and gut fluid [17,18]. SIgA antibodies are the major effectors of mucosal immunity. Secretory immunity is the most desirable barrier defending against mucosal infections preventing initial pathogen colonization by providing SIgA antibodies, which can clear pathogenic bacteria and viruses at mucosal surfaces and neutralize endotoxin within epithelial cells without causing tissue damage [19].
Many research works have been carried out worldwide to explore the effect of diclazuril against E. tenella [20-24]. Our previous study reported that diclazuril effectively alleviated the damage in the cecum induced by E. tenella, which can be promoted by improving the host mucosal immune system in the intestinal tract [25]. However, further investigation of how diclazuril affects BF immune function, especially in chickens infected by E. tenella is a topic under investigation. The purpose of this study was to elucidate the effect of diclazuril on BF morphology and SIgA expression in chickens infected with E. tenella, thereby provide a theoretical basis for coccidiosis control in immune strategy and reduce the economic losses in the poultry industry.
MATERIALS AND METHODS
Preparation of inoculum
Oocysts of E. tenella were propagated, isolated, sporulated, and kept in 2.5% potassium dichromate solution. Sporulated oocysts were washed with distilled water and counted using a cytometer prior to inoculation.
Experimental drug
Diclazuril (>99%, product no: 20080812) provided by Shanghai Veterinary Research Institute, Chinese Academy of Agricultural Sciences (Shanghai, China) was administered through the diet at a concentration of 1 mg/kg.
Experimental chickens
One-day-old Chinese Yellow Broiler male chickens (n=160) were purchased from the hatchery of Huizhong, Luoyang, China, and reared for 14 days in coccidiosis-free conditions. The chickens were kept on wire-floored batteries, fed a commercial prepared diet without any anticoccidial medication, and allowed free access to drinking water. The experiment protocol conformed to the guidelines of the Institutional Animal Care and Use Committee of China (No. Ykz1992.24).
Experimental design
As in our recent report [25], 14-day-old chickens (n=160) were randomly assigned to 4 groups of 40 chickens each. The 4 test groups were as follows: (1) chickens challenged with distilled water as a sham inoculation and not treated with either E. tenella oocysts or diclazuril; this was the normal control group; (2) chickens not inoculated with E. tenella oocysts (prepared as described above) but were treated with diclazuril; representing the diclazuril group; (3) chickens inoculated with 8×104 E. tenella sporulated oocysts/chicken but were not treated with diclazuril; representing the infected and untreated group; (4) chickens inoculated with 8×104 E. tenella sporulated oocysts/chicken and also administrated a regular diet containing 1 mg/kg diclazuril continuously from the 96th to 120th hr for 24 hr after inoculation; this was the infected and treated group.
Histology detection
On the 120th hr, the BF samples were fixed in 4% paraform solution, paraffin-embedded, and sectioned at 5 μm thickness prior to hematoxylin and eosin (H-E) staining or immunohistochemistry for mouse monoclonal antibodies against IgA heavy chain of chicken origin (1:200) (Santa Cruz Biotechnology, Santa Cruz, California, USA) as described previously [25]. Signal was detected using biotinylated goat anti-mouse IgG and DAB kit (Wuhan Boster Biological Technology Ltd., Wuhan, China).
Transmission electron microscopy observation
For transmission electron microscopy (TEM), the BF tissues were fixed in 2.5% glutaraldehyde in PBS at pH 7.4, subsequently postfixed with 1% osmium tetroxide, and washed with PBS. They were then dehydrated in a graded acetone series and saturated in acetone. After embedding in aradite resin, 60 nm thick sections were processed and stained with uranyl acetate and lead citrate, and then examined with an H-7500 TEM (Hitachi, Tokyo, Japan).
Statistical analysis
Statistical analyses were performed by the Student’s t-test. Data were expressed as the mean±SD. P <0.01 was considered statistically significant.
RESULTS
Morphological findings
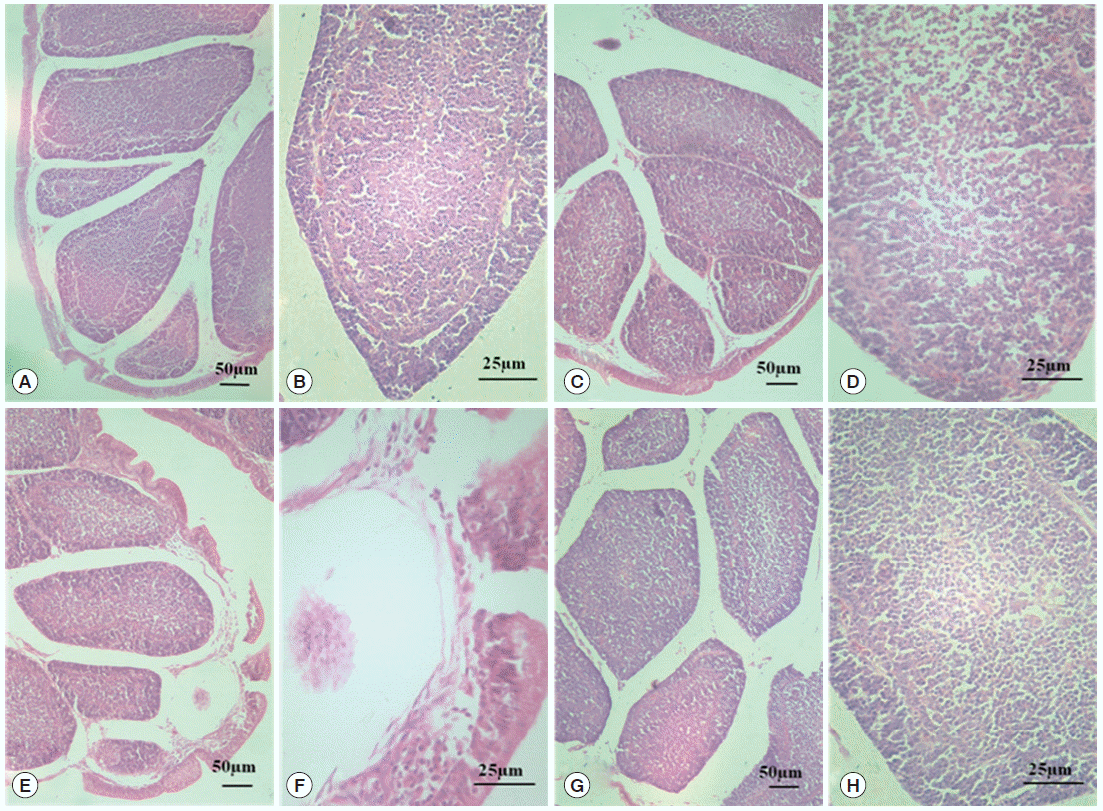
Morphological changes in the BF are shown in Fig. 1. In the normal control group and diclazuril group, the morphology of BF tissues was normal, and the plicae of BF were complete as shown by H-E staining. Well ordered lymphoid follicles clearly separated into cortical and medullary zones by connective tissues, and there were affluent lymphocytes in the medulla. In addition, the interspace between follicles was small (Fig. 1A-D). When chickens were infected by E. tenella, the structure of BF was destroyed. The breaks occurred in the plical epithelium of BF, and lymphoid follicles were disorganized and sparsated. On top of that, the limit between the medulla and the cortex was vagued as well as a reduction in the number of lymphocytes in the medulla (Fig. 1E, F). With the treatment of diclazuril, the plical epithelium of BF became thin, but no breaks; lymphoid follicles organized in order and tight; the limit between the medulla and cortex was also comparatively distinct (Fig. 1G, H).
Ultrastructure changes
Fig. 2 shows the ultrastructural changes in BF cells. In the normal control group, the circumscriptions among ordered cells were clear. The BF cells have an ovoid or sometimes irregular nucleus with nucleoli occupying the central position. In a less dense cytoplasm, we observed vesicles with a variably electron dense granular content (Fig. 2A) with no obvious changes in the diclazuril group (Fig. 2B). Compared with the normal control group, the BF cells decreased in volume and became irregularly arranged, along with associated morphological changes such as reduced amounts of chromatin, which was condensed and compressed against the nuclear envelope and aggregated into large dark, compact masses. This intercellular space also was obviously enlarged (Fig. 2C). With the administration of diclazuril, the injury to the cells lessened and partially injured cell-membranes become visible compared with the infected and untreated group (Fig. 2D).
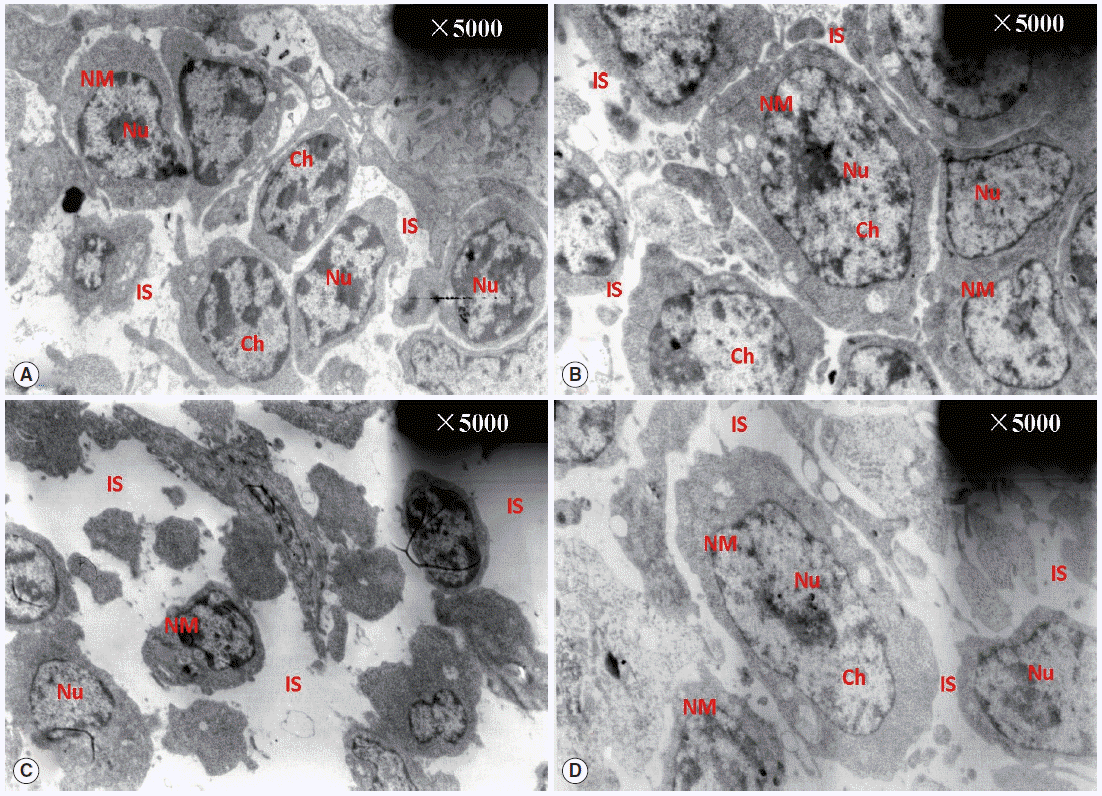
TEM observation of BF. The circumscriptions among ordered cells were clear. The BF cells have an ovoid or sometimes irregular nucleus with nucleoli occupying a central position in the normal control group (A). There are no any obvious changes in the diclazuril group (B). Compared with the normal control group, the BF cells became smaller in volume and even irregularly arranged in the infected and untreated group (C). With the administration of diclazuril, the injury of cells was lessened and partially injured cell-membranes became visible in the infected and treated group (D). Nu, nucleus; NM, nuclear membrane; Ch, chromosome; IS, intercellular space.
SIgA expression in the BF
As can be seen in Fig. 3, SIgA positive cells were mainly present in the epithelium mucosae and the lymphoid follicular cortex, and relatively scarce in the lymphoid follicular medulla in the BF. SIgA was only present in a few cells of the follicular cortex in the normal control group (Fig. 3A, B) and diclazuril group (Fig. 3C, D). For chickens in the infected and untreated group, compared to the normal control group, the positive cells that secrete SIgA were increased significantly by 350.4% (P <0.01) (Fig. 4), not only in the epithelium mucosae but were also found in the cortex and medulla of lymphoid follicles (Fig. 3E, F). Relatively fewer positive SIgA cells can be seen in the epithelium and the cortex with regard to the infected and treated group (Fig. 3G, H), in which the level of SIgA expression was significantly decreased by 46.7% (P <0.01) compared with the infected and untreated group (Fig. 4).
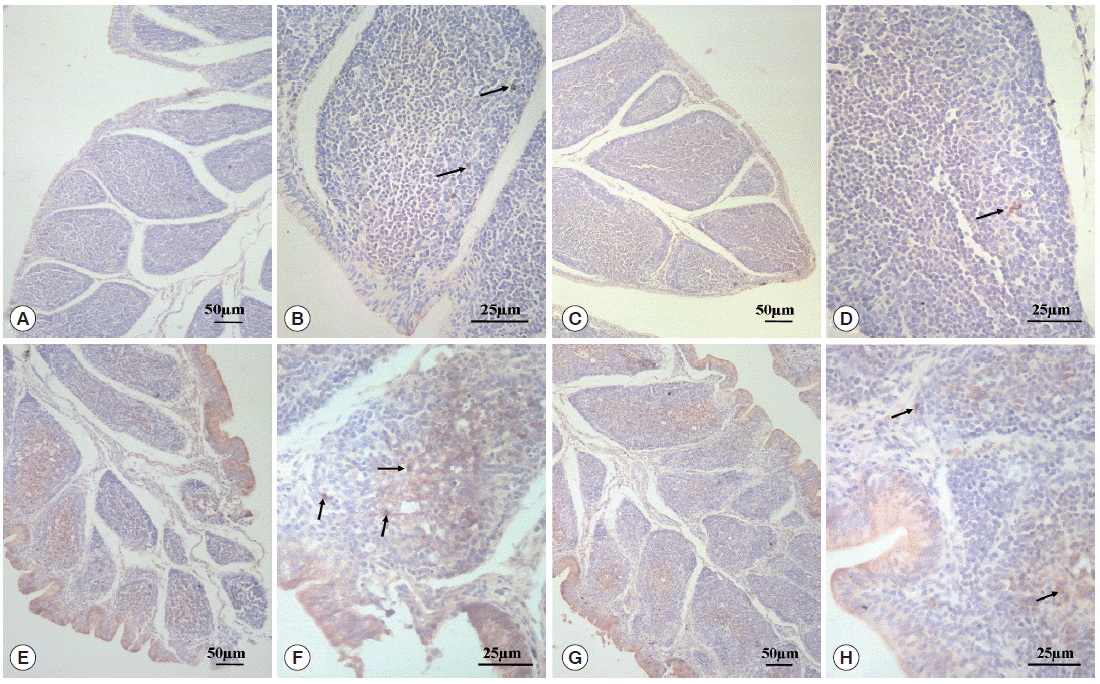
SIgA expression analysis by immunohistochemistry. In the normal control group (A, 100× and B, 400×) and the diclazuril group (C, 100× and D, 400×), only a few SIgA positive cells were distributed in the lymphoid follicular cortex. In the infected and untreated group, SIgA positive cells were present in the epithelium mucosae and the cortex and medulla of lymphoid follicles (E, 100× and F, 400×). Relatively fewer positive SIgA cells were seen in the epithelium and the cortex in the infected and treated group (G, 100× and H, 400×). Arrows indicate the SIgA positive cells.
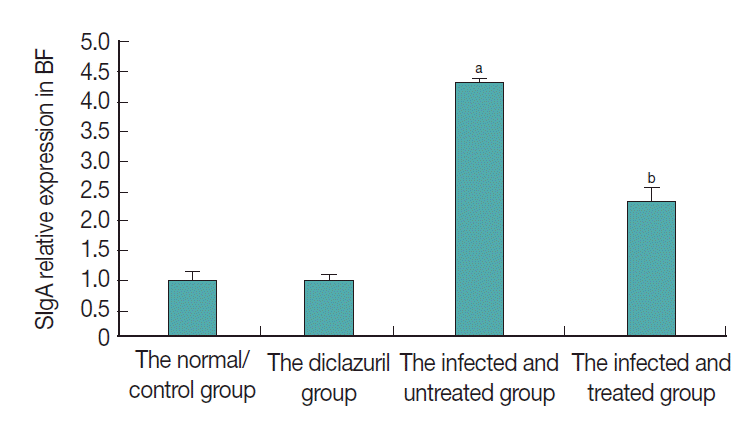
SIgA relative expression in BF. aP<0.01: compared to the normal control group, the positive cells that secrete SIgA were increased significantly by 350.4% in the infected and untreated group. bP<0.01: With the treatment of diclazuril, SIgA expression was significantly decreased by 46.7% compared with the infected and untreated group.
DISCUSSION
The bursa, an epithelial and lymphoid organ, which is found only in birds, plays a protective role in defending avians from pathogenic microorganism infections. The bursa develops as a dorsal diverticulum of the proctadael region of the cloaca. The luminal (interior) surface of the bursa is plicated with as many as 15 primary and 7 secondary plicae or folds. These plicae have hundreds of bursal follicles containing follicle-associated epithelial cells, lymphocytes, macrophages, and plasma cells. Lymphoid stem cells migrate from the fetal liver to the bursa during ontogeny. In the bursa, these stem cells acquire the characteristics of mature, immunocompetent B cells, then to produce specific antibodies to complete a specific immune response.
To give a better interpretation of the effect of diclazuril on the BF morphology and ultrastructure in chickens infected with E. tenella, the morphology and ultrastructure of the BF were observed. We found that E. tenella infection induced obvious damage to the structure of BF. What we observed was the plical epithelium of BF becomes thin and broken. Lymphoid follicles were disorganized and sparsated. The cortico-medullary layer became vague. For the changes of ultrastructure, the cells were concentrated, cracked, and karyopyknotic, and chromatin margination and instercellular space were enlarged. This might be due to E. tenella infection, which could cause serious disorders of the digestive function.
The BF, as the prolongation of the digestive tract was easily affected by protozoan parasites and produced pathological changes [26,27]. E. tenella infection could also lead to decreased resistance and lower anti-oxidant ability in the host. Therefore, pathological changes of BF also probably attributed to lipid peroxidative damage caused by E. tenella infection [28], just as the report that the anti-oxidant ability was decreased in caprine coccidiosis [29]. Diclazuril can improve the pathological changes of BF and anti-oxidant ability of the chickens by inhibiting the growth and reproduction of E. tenella [20,21]. As a result, the injury to the BF was alleviated.
SIgA, a vital factor involved in the intestinal mucosal immunity, plays a key role in protecting the host against a variety of pathogenic infections, such as bacteria, viruses, and parasites. So far, many papers have reported SIgA playing a key role in inhibiting human intestinal parasitic infections [30-32]. Bourguin et al. [33] applied cholera toxin to enhance the intestinal secretory IgA level to defend against Toxoplasma gondii. Del L Yácono et al. [34] also found that SIgA provides immune protection against Toxoplasma in mucosal tissues. SIgA is produced by plasma cells and then secreted to the surface of mucous membrane, such as the intestinal tract and the respiratory tract [18,35]. Birds usually respond to E. tenella infection by cellular immunity and endogenous antibody production [36,37]. The BF is a unique primary lymphoid organ in birds and is responsible for differentiation and maturation of B-cells [38]. When the BF is infected by antigens, B-cells differentiate into plasma cells and secrete antibodies to protect the host [39]. In our study, the secretion of SIgA in BF was significantly increased by 350.4% (P <0.01) after E. tenella infection compared with the normal control group, which only has several positive cells. These findings demonstrated that the BF immune function was triggered by the infection of E. tenella in the parasitized site in cecal tissues, and this stimulated the B-cell differentiation into plasma cells and secreted the SIgA antibody against E. tenella infection. Diclazuril is prone to decrease the number of coccidians and alleviate the pathogenic infections on the host; therefore, the level of SIgA expression was significantly decreased by 46.7% (P<0.01) compared with the infected and untreated group.
Furthermore, an interesting phenomenon was found in this study which was the spatial distribution of SIgA expression in the BF tissue with the treatment. SIgA positive cells were mainly located in the cortical region of the follicles in the normal control group, but they were predominantly distributed along the epithelium of plica after E. tenella infection. When the infected chickens were treated by diclazuril, the immunoreactive cells were mostly detected between the epithelium of plica and cortical region of the follicles. Most of B-lymphocytes are in the cortex of the follicles for 20 days old chicks [40]. Moreover, the function of B-lymphocytes is activated with the antigen invasion [41]. Subsequently, B-lymphocytes differentiate into plasma cells and migrate to the secondary lymphoid organs and tissues [42]. Therefore, we speculate that E. tenella infection not only promoted the maturation of B-cells in BF but also induced their migration. At the same time, diclazuril administration partially reversed the distribution of B-cells, and the SIgA expression was observed between the epithelium of plica and cortical region of the follicles in the infected and treated group. There may be other mechanisms of action, which require further research.
In conclusion, the results of the present study have demonstrated that E. tenella infection in chickens induced obvious damages in BF morphological structure and stimulated the SIgA expression in the BF. The diclazuril treatment effectively alleviated the adverse effects and provided an immune strategy to control coccidiosis by improving the potential immune functions in chickens.
Acknowledgements
This research was sponsored by the China National Natural Science Foundation (grant nos. 31472238 and 31101855) and by the Henan University of Science and Technology Foundation for leaders of Discipline and Technique in Science (20140706).
Notes
We have no conflict of interest related to this work.