Antiplasmodial and Cytotoxic Activities of Toad Venoms from Southern Amazon, Brazil
Article information
Abstract
The drug-resistance of malaria parasites is the main problem in the disease control. The huge Brazilian biodiversity promotes the search for new compounds, where the animal kingdom is proving to be a promising source of bioactive compounds. The main objective of this study was to evaluate the antiplasmodial and cytotoxic activity of the compounds obtained from the toad venoms of Brazilian Amazon. Toad venoms were collected from the secretion of Rhinella marina and Rhaebo guttatus in Mato Grosso State, Brazil. The powder was extracted at room temperature, yielding 2 extracts (RG and RM) and a substance (‘1’) identified as a bufadienolide, named telocinobufagin. Growth inhibition, intraerythrocytic development, and parasite morphology were evaluated in culture by microscopic observations of Giemsa-stained thin blood films. Cytotoxicity was determined against HepG2 and BGM cells by MTT and neutral red assays. The 2 extracts and the pure substance (‘1’) tested were active against chloroquine-resistant Plasmodium falciparum strain, demonstrating lower IC50 values. In cytotoxic tests, the 2 extracts and substance ‘1’ showed pronounced lethal effects on chloroquine-resistant P. faciparum strain and low cytotoxic effect, highlighting toad parotoid gland secretions as a promising source of novel lead antiplasmodial compounds.
INTRODUCTION
The 2015 Nobel Prize in Physiology or Medicine was awarded to 3 scientists who made pioneering discoveries of drugs currently used to treat neglected tropical diseases [1]. A portion of the award was to Youyou Tu (China), guided by ancient Chinese texts, isolated a component from the plant Artemesia annua, called artemisinin, which proved to be highly effective against the malaria parasite [2]. Artemisinin has now become the drug of choice for the treatment of malaria throughout most of the tropical world.
Malaria remains an important cause of illness and death in children and adults in underdeveloped countries in which it is endemic. The drug-resistant malaria parasite is the main problem in the disease control. Artemisinin resistance has been described in malaria endemic areas, threatening malaria control, treatment, and elimination efforts worldwide [3]. World Health Organization (WHO) updated their treatment policy in countries where P. falciparum parasite is endemic from use of monotherapy with drugs such as chloroquine, amodiaquine, and sulfadoxine–pyrimethamine (SP) to currently recommended artemisinin-based combination therapies (ACT) [4]. However, resistance to ACTs has arisen recently in P. falciparum in South East Asia [4,5]. Therefore, it is necessary to develop novel drugs for malaria therapy, particularly in regions where resistant Plasmodium strains are present.
The use of natural products has provided a prospective strategy for identifying novel antimalarial drugs. In this context, the bioprospecting of secondary metabolites can be an important tool to new antimalarial drugs discovery. The use of animals, plants, fungi, and bacteria are important sources of biologically active substances with structural diversity and novel mechanisms of action, which can possibly provide patentable products [6-12]. An example is the development of Captopril®, this medicine was based on research on small peptides from the venom of the South American snake (Bothrops jararaca) that were known to potentiate the action of bradykinin [13-15].
In the class of Amphibians are the family Bufonidae that possesses about 471 species [16]. An important genus of this family is Rhinella (formerly Bufo in the New World), which consists of about 258 species. In Latin America, they are found in Amazon regions of Brazil, Bolivia, Colombia, Peru, Suriname, Guiana, and Venezuela [17]. The skin secretions and venom of amphibians are rich sources of bioactive compounds, such as peptides, alkaloids, bufadienolides, biogenic amines, and proteins. These molecules play a crucial role in the physiological functions of these animals, especially for predation and protection against microorganisms [18,19]. Previous studies with Rhinella marina venom resulted in the identification by LC-MS of the 4 bufadienolides; telocinobufagin, marinobufagin, bufalin, and resibufogenin, and in Rhaebo guttatus venom marinobufagin was identified [20]. Bufadienolides are an important group of steroid hormones that has shown vasoconstriction [21], antiviral [22,23], antitumoral [24-26], cytotoxic [8,20,27,28], antileishmanial, and antitrypanosomal activities [29].
The potential of natural products to provide the development of antimalarial compounds is evident [30]. Animals contain a large assortment of structurally unique secondary metabolites that can be useful as new chemical templates for drug discovery [6,8]. Although amphibian skin secretions have proved to be a rich source of exclusive molecules, no studies have evaluated the potential antimalarial activity of these compounds. Our objectives were to conduct bioprospecting of the extracts and pure compound from R. marina (synonymy Bufo marinus) and R. guttatus toad venom, occurring in the Southern Amazon of Mato Grosso, Brazil, with antiplasmodial and cytotoxic activities against tumor and normal cells lines.
MATERIALS AND METHODS
Sample collection
Toad venoms were collected from the secretion of R. marina and R. guttatus in Mato Grosso State, Brazil. The animals were identified by one of the authors (D. J. Rodrigues, IBAMA, SISBIO: no. 30034-1). Voucher specimens (R. marina ABAM-H 1262 and R. guttatus ABAM-H 1538) were deposited in the Acervo Biológico da Amazônia Meridional (Sinop, Mato Grosso, Brazil).
Extraction and isolation
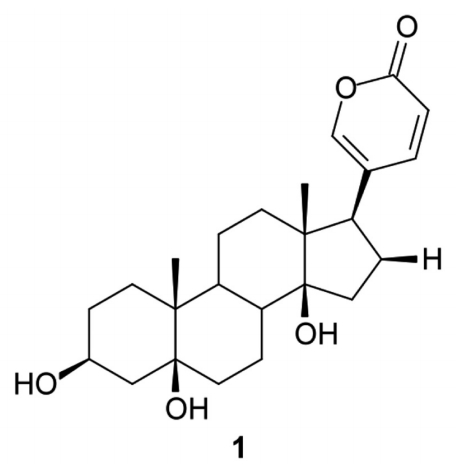
Samples of toad venoms of R. marina and R. guttatus were dried, powdered, and extracted 3 times (5 ml) with CHCl3/MeOH by ultrasonication for 10 min at room temperature [20]. The extracts were identified by the following codes: RM (R. marina) and RG (R. guttatus). The extract (1.1 g) from R. marina venom was chromatographed on Sephadex LH-20 column (GE Healthcare, Freiburg, Germany) using MeOH as eluent. The subfraction 140 yielded the compound ‘1’ (25.7 mg). The compound ‘1’ (Fig. 1) was identified by spectrometric methods (mass, NMR 1H and 13C) and by comparison with literature [18], as a bufadienolide, named telocinobufagin.
Parasites
Chloroquine (CQ)-resistant W2 strain of P. falciparum was used for in vitro blood stage culture to test the antiplasmodial efficacy of toad venom extracts and ‘1’. P. falciparum continuous culture was maintained according to the method described previously [31] with minor modifications. Cultures were maintained at 5% hematocrit using type O+ human erythrocytes in RPMI 1640 medium (Sigma-Aldrich, St. Louis, Missouri, USA) supplemented with 25 mM NaHCO3, 1.0% albumax, 45 mg/L hypoxanthine, 40 μg/ml gentamycin and incubated at 37˚C under approximately 5% of CO2. The parasites were synchronized at ring stage by sorbitol treatment [32]. Initial parasitemia was adjusted to 0.5% with 2% hematocrit in experiments.
Antiplasmodial activity
Growth inhibition of intraerythrocytic forms and parasite morphology were evaluated in culture by the microscopic observation of Giemsa-stained thin blood films. After synchr onization by sorbitol treatment, parasite culture at ring stage (0.5% parasitemia and 2% hematocrit) was added to each well of 96-well microculture plates. The toad venom extracts (RG and RM) and ‘1’ were dissolved in DMSO and diluted to appropriate concentrations using complete medium (10 mg/ml) and stored at 4˚C. The final concentration in the culture was 1%. After incubation at 37˚C for 48 hr, P. falciparum growth inhibition was assessed by Giemsa-stained smears by observing 5,000 erythrocytes per 1 thin blood film in triplicate. The culture medium was replaced with fresh medium with or without test samples/control drugs. Chloroquine (CQ) was used as a reference antimalarial. The activity of the extracts and ‘1’ was expressed as the percentage reduction in parasitemia relative to controls without drugs. All experiments were performed in triplicate. The results were expressed as the mean of the IC50 (the lethal drug concentration that reduced parasite viability in 50%).
Cells culture to cytotoxic assays
The cytotoxicity testing was performed in 2 cell lines; human hepatoma cells (HepG2) and normal kidney glomerular cells (BGM). Both of these cell lines were cultured in 75 cm2 sterile flasks. RPMI 1640 (Sigma-Aldrich, St. Louis, Missouri, USA) medium supplemented with 10% heat-inactivated fetal calf serum and 40 mg/L gentamycin in a 5% CO2 atmosphere at 37˚C. When confluent, the cell monolayer was washed with culture medium, trypsinized, distributed in a flat-bottomed 96-well plate (5×103 cells/well), and incubated for 18 hr at 37˚C for cell adherence [33]. The compounds (20 μl) were diluted to various Log concentrations (0.2-200 μg/ml) and incubated with the cells for 24 hr in a 5% CO2 atmosphere at 37˚C.
Cytotoxic activity
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution (5 mg/ml; 20 μl/well) was added to evaluate mitochondrial viability; after a further 3 hr incubation, the supernatants were carefully removed, 100 μl of DMSO was added to each well, and the reactions were mixed to solubilize the formazan crystals. The optical density was determined at 540 nm to measure the signal and background, respectively (Spectra Max340PC384, Molecular Devices, Sunnyvale, California, USA). The cell viability was expressed as a percentage of the control absorbance in the untreated cells after subtracting the appropriate background.
The neutral red assay (NR) [34] was also performed in triplicate. After addition of the samples and 3 hr incubation, the supernatants were carefully removed and 200 μl of neutral red solution (40 μg/ml) were added to each well. The microplates were incubated again at 37˚C in a humidified atmosphere with 5% CO2 for 3 hr. Then, the supernatant was removed and added to 200 μl of formaldehyde solution (0.5%, v/v) and CaCl2 (1%) to each well of the test plate. After 5 min, the supernatant was again removed, and 100 μl acid alcohol solution (50%, v/v ethanol in 1% v/v acetic acid) were added to each well. The absorbance of the microplate was read in an ELISA reader (Spectra Max340PC384, Molecular Devices, Sunnyvale, California, USA) using 540 nm filter.
The minimum lethal dose for 50% of the cells (LD50) was determined as described [35]. RM and RG extracted compounds, ‘1’, and chloroquine concentrations used to calculate LD50 were 2-2,000 μg/ml.
Selectivity index (SI)
A selectivity index (SI) corresponding to the ratio between the cytotoxic and antiparasitic activities of each sample tested. The values greater than 10 were considered indicative of lack of toxicity; however, the substances with values below 10 were considered toxic [36]. The SI index was calculated as follow: SI=LD50 Cell/IC50 P. falciparum.
Statistical analysis
The concentrations of compounds able to inhibit 50% of parasite growth (IC50) were determined based on the equation of the curve obtained by plotting the % of parasitemia regression vs the log of the concentration of compound. The coefficients of regression of these curves were calculated using the method of least squares. The LD50 were determined based on the equation of the curve obtained by plotting the % of cellular death versus the concentration of compound (GraphPad Prism SoftwareÒ, version 5.0 for Windows, San Diego, California, USA). The average IC50 and LD50 were compared using ANOVA. Statistical significance was defined at the 5% level (P<0.05).
RESULTS
In vitro anti-plasmodial activity
The extracts RG, RM, and ‘1’ diluted in DMSO were assayed for antiplasmodial activity against chloroquine-resistant P. falciparum W2. Table 1 shows the antiplasmodial activity of RG, RM, and ‘1’ in 2 different experiments. When evaluated the venom extracts and ‘1’ in concentrations of 100 μg/ml, all of them showed the parasitemia reduction above 60%. The compound ‘1’ showed and high potential of parasitemia reduction in vitro and the lower standard deviation (Fig. 2). Despite the high reduction of parasitemia of compound ‘1’, RG extract had a lower IC50 value. Starting from 100 μg/ml, the compounds were diluted to various concentrations (0.78-100 μg/ml) to calculate the IC50 values. All samples (RG, RM, and ‘1’) showed values lower than 2 μg/ml, thus considered active against P. falciparum W2 strain (Fig. 3).

IC50, LD50, and SI values obtained from in vitro tests with extracts (RG and RM), 1 obtained from toads venom, and chloroquine (CQ) against P. falciparum W2 strain
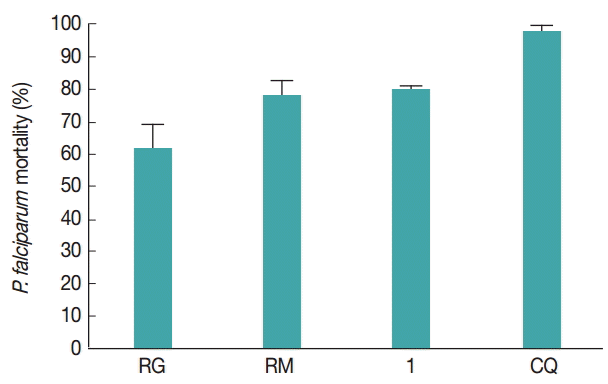
Parasitemia reduction (%) of P. falciparum W2 strain showing the mean±SD of the RG, RM, and ‘1’ compound. RM, R. marina; RG, R. guttatus; ‘1’, telocinobufagin at a concentration of 100 µg/ml; CQ, chloroquine, the antimalarial used as the positive control at a concentration of 100 μg/ml.
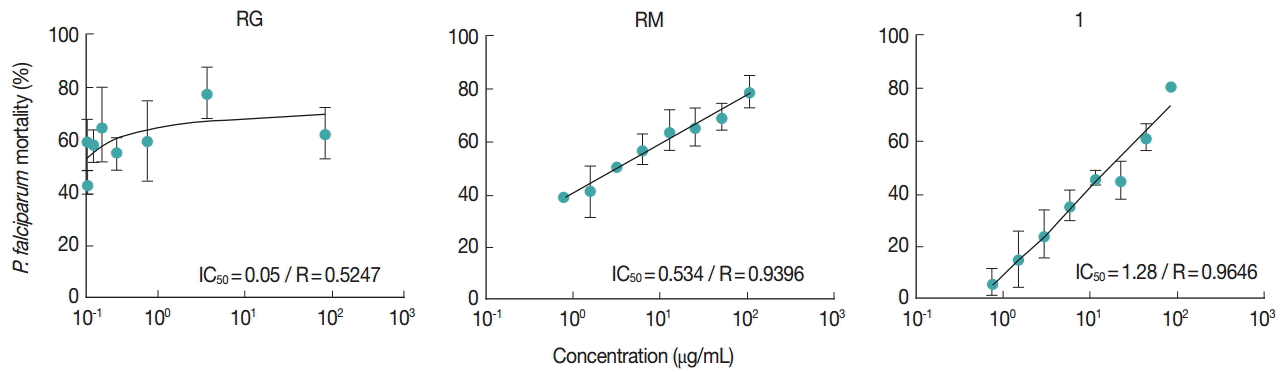
Dose-response curves of RM, RG, and ‘1’ in function of linear regression. Effects of different concentrations of RM, RG, and ‘1’ (0.78-100 μg/ml) on the activity of P. falciparum W2 strain. Each point represents the mean±SD of 2 different samples performed in triplicates. IC50 values are presented in graphic.
Cytotoxic activity on HepG2 and BGM cells
To evaluate the cytotoxic activity of the extracts and compound ‘1’, cytotoxic assays were performed by MTT and neutral red assays in human hepatoma cells (HepG2) and normal kidney glomerular cells (BGM). When analyzed the cytotoxic activity of the samples by MTT assay in HepG2 cells, it was observed that all samples (RG, RM, and ‘1’) showed high LD50 values, being low or not cytotoxic to this cell line (57~ >100 μg/ml) (Table 1). In MTT assay in BGM cells, RG extract showed LD50 values of 34.8 μg/ml. This value to RG extract can be considered low cytotoxic to BGM cells. The other extracts (RM and ‘1’) showed high LD50 values (38.9~>100 μg/ml), not being cytotoxic to BGM cells.
When the cytotoxic activity of the samples was analyzed in the neutral red assay, it was observed that all compounds showed high LD50 values in both the cell line HepG2 and is therefore considered not cytotoxic. In BGM cells, the RG showed LD50 values of 22.6 μg/ml (Table 1).
Evaluating the selectivity index (SI), all compounds showed high selectivity for the parasites when analyzed by MTT assay in the HepG2 cells (SI>72.8). In BGM lineage, it was observed SI>100 for the all compounds tested. In neutral red assays, all samples showed high selectivity for the parasites when analyzed in HepG2 e BGM cells (>100) (Table 1).
DISCUSSION
Several extensive molecules and natural products screens against P. falciparum blood stages have been published annually, emerging as new candidates for antimalarial drugs. However, no antiplasmodial activity of molecules and aqueous and organic extracts obtained from toad skin glands, whose secretions exhibit bufadienolides, was previously described.
Secretions from 2 toad species, R. marina and R. guttatus, were chemically investigated previously, and the isolation of telocinobufagin was described in R. marina venom [20]. When analyzed the IC50 values of extracts and compound ‘1’ from the toad venoms, it was noted that all samples showed values lower than 5 μg/ml can be considered highly active against P. falciparum W2 strain. According to WHO guidelines and studies by Pink et al. [37], the rating for the antimalarial activity of a drug is as follows: highly active (IC50 <5 μg/ml), promising active (5-15 μg/ml), lowest active (15-50 μg/ml), and inactive (>50 μg/ml).
The telocinobufagin (‘1’) showed a high reduction of parasitemia in vitro. One study demonstrated the biological activity of steroids telocinobufagin and hellebrigenin against Leishmania chagasi promastigotes, but only hellebrigenin was active against Trypanosoma cruzi trypomastigotes [29]. Ferreira et al. [20] showed the differences in composition between R. marina and R. guttatus venoms, in terms of the number and type of constituents. The telocinobufagin is found only in R. marina venom, together with another 3 bufadienolides: marinobufagin, bufalin, and resibufogenin. However, only bufadienolide (marinobufagin) was identified in R. guttatus venom. Thus, the lowest IC50 value in antiplasmodial test can be explained by the presence of only on bufadienolide in the constitution of R. guttatus venom. This suggests a synergistic effect of bufadienolides present in R. marina venom.
Venom extracts from R. marina and R. guttatus showed moderate cytotoxic activities against HepG2 and BGM cells in MTT assay, respectively. Besides, when the cytotoxic activity of the extracts was analyzed by neutral red assay, it was observed that all samples showed high LD50 values in the HepG2 cells, and RG showed moderate cytotoxicity in BGM cells. A study in seaweed in northeastern Brazil showed greater susceptibility of BGM cells to crude extracts of the studied algae when compared to macrophages [37]. However, in our study, the purified substance telocibufaginin did not show cytotoxic activities for cell line HepG2. The low cytotoxicity of the telocinobufagin against cancer cell lines (HL-60, SF-295, MDA-MB-435, and HCT-8) was demonstrated in bufadienolides isolated from the Brazilian toad Rhinella schneideri [8].
In our study, antiplasmodial and cytotoxic activities in HepG2 cells were best observed in RG extract. The presence of 4 bufadienolides in RM extract displayed less in vitro efficacy against P. falciparum compared to the RG extract. These results differ from previous studies that have reported a higher cytotoxic activity of venom extracts from R. marina in comparison to those from R. guttatus due the presence of 3 other bufadienolides (telocinobufagin, bufalin, and resibufogenin), as well as marinobufagin, only bufadienolide identified in R. guttatus venom [20].
According to Bézivin et al. [36], values higher than 10 (SI>10) is indicative of high selectivity values, whereas values below 10 (SI<10) are considered as low selectivity. In this study, for all tested samples (RG, RM, and ‘1’), the SI was considered as high selectivity. The high selectivity index of the extracts and ‘1’ indicate either an absence or large difference in the structure of the target molecule between P. falciparum and the mammalian cell. Therefore, these extracts could be exploited to identify the molecular target, which subsequently could be helpful to design novel therapeutics to combat malaria.
In this work, it was important to assess the cytotoxic activity of extracts and pure compound ‘1’ in 2 different cell lines; tumoral and non-tumoral cells. An important article showed recently that the same chemical agent or compound can have different mechanisms of action in the cells when tested in different cell types [39]. The RM and ‘1’ compounds did not have the LD50 values determined in BGM cells used in both cytotoxic assays. Thus, it can be suggested that the mechanism of action of these compounds in BGM cells can be different from HepG2 cells. These results demonstrate the importance of the cytotoxicity test and calculating the selectivity index for natural products with possible antimalarial activity. If only IC50 values for parasite were analyzed, it may be concluded that all the samples would have potential antimalarial acticities. In a study by Cenzi [40] to assess the antimalarial activity of sulfonamides derived from 4-metoxichalcona, all molecules were considered highly active against P. falciparum W2. However, when analyzing the selectivity index values of sulfonamides derived from 4-metoxichalcona, it was concluded that none of molecules was considered to have high selectivity for the parasites.
In summary, all assayed samples (extracts and ‘1’) showed antiplasmodial activity. The extracts of R. marina and R. guttatus venoms and ‘1’ isolated compound from R. marina showed pronounced lethal effects in P. faciparum chloroquine-resistant strain, highlighting toad parotoid gland secretions as a promising source of novel lead antimalarial compounds. The compound ‘1’ can serve as a prototype molecule for the development of more active compounds.
Acknowledgements
This work was financially supported by the CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brazil) and FAPEMAT (Fundação de Amparo à Pesquisa do Estado de Mato Grosso, Brazil).
Notes
The authors declare no conflict of interest.