A case of symptomatic splenic infarction in vivax malaria
Article information
Abstract
Splenic infarction is a rare complication in malaria cases, and is caused primarily by Plasmodium falciparum. Recently in South Korea, only P. vivax has prevailed since 1993. Although the probability that symptomatic splenic infarction may occur in vivax malaria cases is considered relatively high, there have never been any case reports describing the occurrence of symptomatic splenic infarction in cases of vivax malaria. A 34-year-old man presented with fever that had persisted for 5 days. P. vivax infection was verified using a peripheral blood smear, and chloroquine was utilized to treat the fever successfully. Six days later, the patient developed pain in the left upper abdomen, which was diagnosed as splenic infarction by computed tomography.
INTRODUCTION
Vivax malaria in Korea has a history of more than 500 years. In the period between 1960 and 1980, the National Malaria Eradication Service, in collaboration with the World Health Organization, reported that the number of vivax malaria cases in South Korea had been successfully reduced, and the final cases of indigenous vivax malaria was documented in 1984 (Soh et al., 1985). Although the status of malarial incidence in North Korea remains unknown, it is reasonable to assume that little has changed in this regard. Since the re-emergence of the Plasmodium vivax malaria first reported in South Korea in 1993, vivax malaria has prevailed in regions adjacent to the demilitarized zone (DMZ); this indicates that P. vivax has reemerged from across the DMZ, i.e., from North Korea (Chai, 1999). Consequently, the majority of Koreans in South Korea under the age of 30 tend not to be immune to malaria. Immunity to the disease determines the clinical features of malaria; life-threatening complications are rare in semi-immune patients, but occur commonly in non-immune patients. Thus, complications associated with vivax malaria may occur more frequently in Korea than in other areas in which malaria is endemic.
Splenic infarction associated with malaria is a rare complication. A Medline search on PubMed (http://www.ncbi.nlm.nih.gov/PubMed/) for 'malaria' AND 'splenic infarction' or 'malaria' AND 'spleen' AND 'infarction' results in 9 cases, which have been well summarized by Bonnard et al. (2005), and 1 additional case of splenic infarction, which was detected incidentally during an autopsy (Oga et al., 2001). With the exception of one case of vivax malaria co-infection, all patients in whom Plasmodium was identified were found to have been infected by P. falciparum, rather than P. vivax. Herein, we report a case of symptomatic splenic infarction in a case of vivax malaria.
CASE RECORD
A 34-year-old man visited the Inha University Hospital in Incheon, Korea, complaining of a fever that had persisted for 5 days. Four days prior to the patient's visit, he had undergone ultrasonography at a nearby clinic, and was shown to be suffering from splenomegaly; the patient's spleen was approximately 14 cm long along the greatest axis. As malaria was not yet suspected in this case, medication was prescribed solely for symptomatic relief. At our outpatient department (OPD), fever and palpable splenomegaly were detected, and trophozoites and schizonts of P. vivax were detected at a density of 1.875 × 109/L. A blood examination revealed the following: hemoglobin level, 9.8 mM/L; white blood cell count, 3.8 × 109/L; and platelet count, 47 × 109/L. Serum aspartate aminotransferase, alanine aminotransferase, and bilirubin levels were as follows: 1.77 mM/L, 2.42 mM/L, and 28.9 µM/L, respectively. The patient was prescribed a course of chloroquine (25 mg/kg over 48 hr) and was instructed to take primaquine after the fever had subsided. Six days later, the patient made another visit to the OPD, due to pain in the left upper abdomen, accompanied by pain in the left shoulder. The patient was afebrile, but continued to exhibit palpable splenomegaly. Upon admission, computed tomography (CT) of the abdomen revealed findings consistent with splenic infarction, i.e., multiple areas of low attenuated density in the enlarged spleen (Fig. 1A). Over the 3 days of hospitalization, the patient's other vital signs remained stable. No malarial trophozoites were observed on the blood smear. The patient's hemoglobin level, white blood cell count, and platelet count were 8.0 mM/L, 10 × 109/L, and 336 × 109/L, respectively. Liver function tests and tests for markers of hypercoagulable conditions, such as protein C or antithrombin III, were all normal. Upon discharge, the patient was prescribed a 14-day regimen of primaquine (15 mg/day), coupled with an analgesic that promptly relieved the patient's abdominal pain. 10 weeks later, the patient evidenced no symptoms consistent with splenic infarction, and a follow-up abdominal CT revealed partial resolution of the splenic infarction (Fig. 1B).
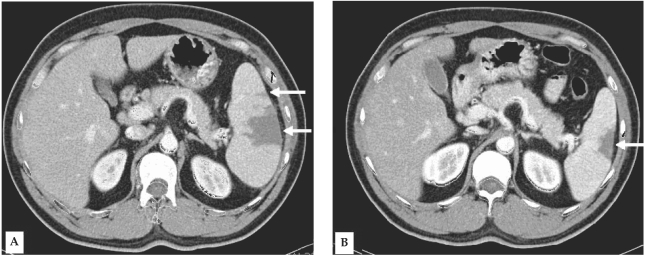
Contrast-enhanced computed tomography (CT) of the abdomen reveals multiple areas of low attenuation density in the enlarged spleen (arrows) (A). Follow-up CT acquired 10 weeks after the initial CT demonstrates partial resolution of the large segmental infarction (arrow), and complete resolution of the small infarcted areas (B).
DISCUSSION
In Korea, since the last report of P. falciparum infections among intraveous drug abusers in the 1950s, no cases of indigenous falciparum malaria have been observed (Chai, 1999). Therefore, after 1993, all reported cases of indigenous malaria with severe complications have been attributed exclusively to infection with P. vivax. With the reemergence of vivax malaria in South Korea, an increasing number of associated complications have been chronicled, including the following: spontaneous splenic rupture (Shin et al., 1999), retinal hemorrhage (Choi et al., 2004), and pulmonary edema and subcapsular splenic hematoma (this was a fatal case) (Park et al., 2005). We have also encountered several cases of pulmonary edema and a case of subcapsular splenic hematoma (in preparation).
Although splenomegaly is frequently observed in malaria cases, it tends not to receive special attention, as it is not usually accompanied by any symptoms, and can be gradually resolved via standard anti-malarial therapy. Pathology of the malarial spleen reveals a variety of characteristic features, including thrombi in the arterioles, veins, and sinusoids, which are frequently associated with hemorrhage, necrosis, and infarction (Hershey and Lubitz, 1948). By way of contrast, clinical splenic complications, including spontaneous splenic rupture, subcapsular splenic hematoma, splenic cyst, splenic abscess, splenic infarction, hyperreactive malarial syndrome, splenic torsion, ectopic spleen, and hypersplenism have only rarely been reported (Zingman and Viner, 1993).
With regard to splenic infarction, the true incidence rate of splenic infarction can easily be underestimated; splenomegaly is determined via radiologic methods only in cases in which malarial patients complain of splenic symptoms, and the frequency with which splenic infarction is associated with these symptoms remains unknown. In fact, in our patient, the splenomegaly was detected two days after the onset of fever, and the magnitude of the splenomegaly was larger than that has been observed in other patients suffering from vivax malaria. During management, we believed the patient's spleen to be unusually large, but we did not attend to his splenomegaly until he complained of pain in the abdomen and left shoulder; we merely cautioned the patient to avoid trauma to the spleen. Similar situations are likely to occur in other countries, particularly in areas in which medical resources are limited. The actual incidence rate of splenic infarction can be determined in cases in which CT or ultrasonography is performed more frequently on malarial patients. For example, 2 cases of asymptomatic splenic infarction chronicles were discovered incidentally during ultrasonography (Agarwal et al., 1997).
Cases of splenic infarction attendant to malaria have been reported principally in cases of falciparum malaria, in which high levels of parasitemia and microvascular sequestration of parasitized red blood cells can constitute predisposing factors (Bonnard et al., 2005). Although these predisposing factors are absent in cases of vivax malaria, the pathology of the spleen observed in vivax malaria patients indicates that splenic infarction occurs frequently in such cases (Hershey and Lubitz, 1948), probably secondary to ischemia induced by hyperplasia of the reticuloendothelial system. Thus, the possibility remains high that clinically overt splenic infarction may occur in cases of vivax malaria. Our case is, to the best of our knowledge, the first documentation of clinically overt splenic infarction in a case of vivax malaria, and examinations for splenic infarction should be included in diagnoses of vivax malarial patients with abdominal symptoms and splenomegaly.
In cases in which splenic infarction is suspected, it can be readily diagnosed by a CT showing multiple wedge-shaped regions of low attenuation, which are distinctively different from those observed on CT images of splenic rupture or subcapsular hematoma (Miller et al, 2004). Ultrasonography is another tool that can be used in the evaluation of splenomegaly, but this technique is less sensitive than CT during the acute stage of infarction. Thus, it remains uncertain as to whether asymptomatic splenic infarction was actually present in our patient on day two. Splenic angiography, if performed, will show wedge-shaped regions of reduced perfusion corresponding to the infarction patterns observed on CT. Splenic abscess can be excluded by radiologic findings and clinical features, as an abscess is normally accompanied by systemic symptoms (Green, 2001). Although the symptoms of perisplenitis are purported to be more severe than those associated with 'usual' splenomegaly (Read et al, 1946), it remains uncertain as to whether this illness is truly different from 'usually symptomatic' splenomegaly.
It may to be more cost-effective to selectively conduct radiologic tests on patients with clinical findings predictive of the presence of splenic infarction in cases of asymptomatic splenomegaly. Although Bonnard et al. (2005) did not specify which clinical features could be used to predict splenic infarction, an extrapolation of the findings of our case demonstrates that the following findings may represent these markers: the early development and magnitude of splenomegaly, and the persistence of splenomegaly and thrombocytosis after recovery from the febrile phase of malaria. At the time of diagnosis, splenomegaly was detected in 50% of the Korean patients 12 days (mean) after the onset of fever, which resolved a few days or weeks after the clearance of parasitemia (Oh et al., 2001). Thus, the early development of splenomegaly and its persistence a few weeks beyond the febrile phase constitute unusual findings in Korean vivax malaria cases. Thrombocytopenia is a frequent observation in vivax malaria cases in Korea (Oh et al., 2001), and tends to resolve 7-14 days after the clearance of parasitemia. Thrombocytosis is observed during the interim phase between thrombocytopenia and the resumption of normal platelet counts (Horstmann et al., 1981). Thus, persistence of thrombocytosis for more than a few weeks after the clearance of fever is suggestive of the presence of focal complications, including splenic infarction. Our assumptions will require further investigations into incidental cases.
Splenic infarction in malarial patients is treated symptomatically. Splenic rupture or splenic abscess has been demonstrated to occur in cases of splenic infarction induced by other diseases (Miller et al., 2004), but until the preparation of this report, there have been no reports showing that malaria-associated splenic infarction necessarily includes any serious complications.