Effect of 2, 6-Dichlorobenzonitrile on Amoebicidal Activity of Multipurpose Contact Lens Disinfecting Solutions
Article information
Abstract
Multipurpose contact lens disinfecting solutions (MPDS) are widely used to cleanse and disinfect microorganisms. However, disinfection efficacy of these MPDS against Acanthamoeba cyst remain insufficient. 2, 6-dichlorobenzonitrile (DCB), a cellulose synthesis inhibitor, is capable of increasing the amoebical effect against Acanthamoeba by inhibiting its encystation. In this study, we investigated the possibility of DCB as a disinfecting agent to improve the amoebicidal activity of MPDS against Acanthamoeba cyst. Eight commercial MPDS (from a to h) were assessed, all of which displayed insufficient amoebicidal activity against the mature cysts. Solution e, f, and h showed strong amoebicidal effect on the immature cysts. Amoebicidal efficacy against mature cysts remained inadequate even when the 8 MPDS were combined with 100 μM DCB. However, 4 kinds of MPDS (solution d, e, f, and h) including 100 μM DCB demonstrated strong amoebicidal activity against the immature cysts. The amoebicidal activity of solution d was increased by addition of DCB. Cytotoxicity was absent in human corneal epithelial cells treated with either DCB or mixture of DCB with MPDS. These results suggested that DCB can enhance the amoebicical activity of MPDS against Acanthamoeba immature cyst in vitro.
Acanthamoeba keratitis associated with contact lenses wearers has been increasing in recent years [1,2]. For cleansing, rinsing, storing, and disinfecting microorganisms, multipurpose contact lens disinfecting solutions (MPDS) are widely used. However, the majority of MPDS retailed in Korea are ineffective against Acanthamoeba, especially the cyst [3]. Acanthamoeba trophozoites can convert itself into highly resistant cyst form, which diminishes the effectiveness of available therapeutic agents [4]. For this reason, new and more efficacious treatment options against cysts have been proposed and are still being examined [5–8].
Cellulose is the main component of the cyst wall [9]. Cellulose synthesis inhibitor 2, 6-dichlorobenzonitrile (DCB) blocked the encystment of Acanthamoeba and improved the anti-amoebic effects [7,10]. In this study, we tested whether DCB can enhance the amoebicidal effects of the MPDS against Acanthamoeba, especially the cysts.
Acanthamoeba castellanii Castellani was obtained from the American Type Culture Collection (ATCC 30011), and axenically cultured in PYG (Protose peptone-Yeast extract-Glucose) medium. Encystation of Acanthamoeba was performed in encystment media [11]. DCB was purchased from Sigma Aldrich. Eight types of MPDS (Table 1) were used to determine amoebicidal activities against Acanthamoeba cyst by the most probable number (MPN) technique [12].
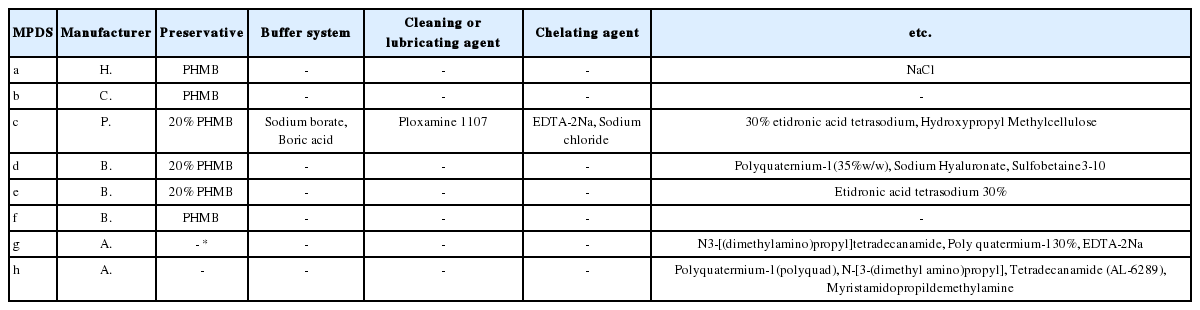
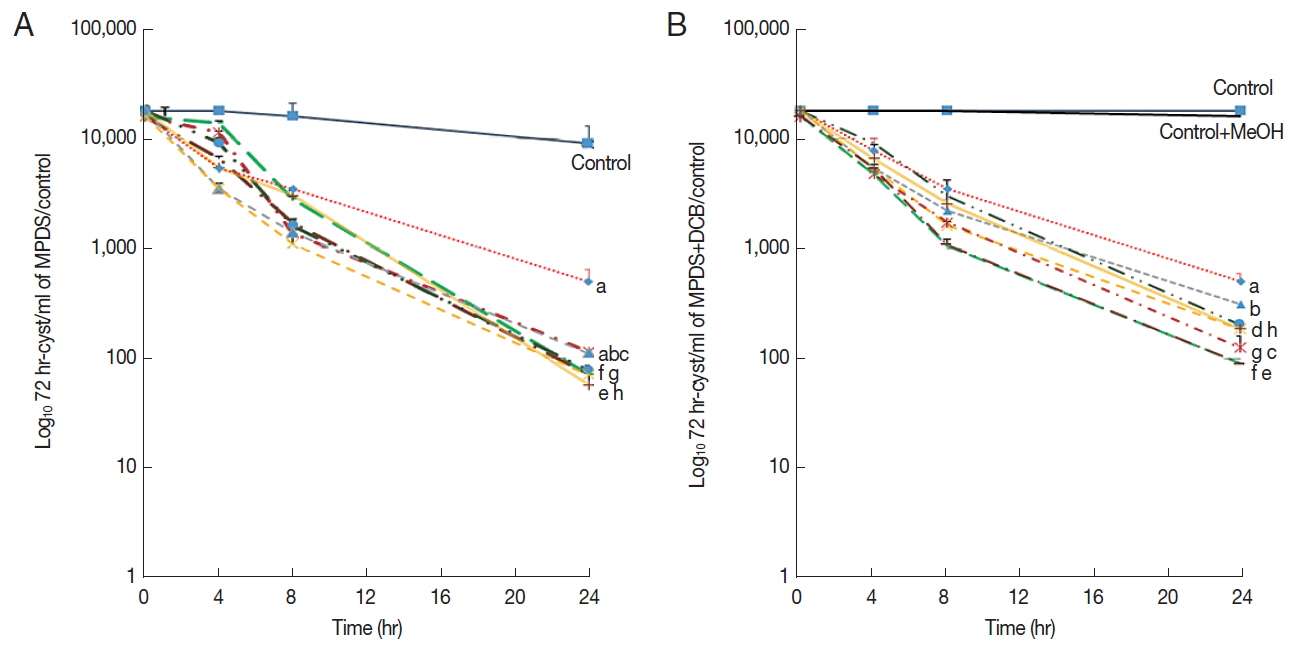
Almost all MPDS showed insufficient amoebicidal activity against the 72 hr-induced mature cysts (Fig. 1A). Even though 100 μM DCB was added to the 8 MPDS, no significant differences were found between MPDS alone and MPDS combined with DCB against the mature cysts (Fig. 1B). The amoebicidal activities of the MPDS with or without DCB were insufficient against the 48 hr-induced cyst (Supplementary Fig. S1). Three of the 8 MPDS (solution e, f, and h) showed sufficient amoebicidal activities against 24 hr-induced immature cysts (Fig. 2A). Combining 100 μM DCB to the 8 commercial MPDS, 4 (solution d, e, f, and h) out of 8 MPDS showed sufficient amoebicidal activities against the immature cysts (Fig. 2B). The amoebicidal activity of MPDS-d was increased against immature cysts upon addition of DCB. MPDS-a combined with DCB displayed slightly increased amoebicidal activity against the immature cyst (Fig. 2B). Encystation ratio revealed 0% mature cysts at 0 hr induction, 27% mature cysts at 24 hr induction, 55% mature cysts at 48 hr induction, and 84% mature cysts at 72 hr induction when incubated in encystation media [13]. We supposed that mature cyst, immature cyst (encysting cyst) and trophozoite were mixed in 24 hr-induced group (Fig. 2), and the DCB acted on immature cyst by blocking its encystation, thereby enhancing the amoebicidal effect on the immature cyst.
A most probable number of Acanthamoeba 72 hr-induced cysts after incubation with 8 different types of MPDS (solution a–h) and the combination of MPDS with 100 μM DCB. (A) illustrates amoebicidal activity of MPDS against 72 hr-induced cysts, and (B) showed that of MPDS combined with 100 μM DCB. Data are presented as mean±SEM from 3 independent experiments.
A most probable number of Acanthamoeba 24 hr-induced cysts after incubation with 8 commercial MPDS (solution a–h) and the combination of MPDS with 100 μM DCB. (A) showed amoebicidal activity of MPDS to 24 hr-induced cysts. (B) showed that of MPDS combined with 100 μM DCB. Data are presented as mean±SEM from 3 independent experiments.
To determine the cytotoxicity of DCB, human corneal epithelial (HCE) cells were treated with 100 μM DCB and 8 kinds of MPDS combined with DCB. Cytotoxicity was assessed visually after Giemsa staining and optical density measurement at 590 nm after 0.1 ml of cells were solubilized in 5% sodium dodecyl sulfate. Percent cytotoxicity was calculated according to the following formula: % cytotoxicity=100−[(OD of the experimental well−OD of HCE cell alone)/OD of control cells]× 100. Compared to the control solution, 100 μM DCB did not cause cytotoxicity to HCE cells after 30 min of incubation (Fig. 3). Most MPDS combined with DCB showed no significant cytotoxicity towards HCE cells (Fig. 3). Minor levels of cytotoxicity was detected in HCE cells treated with MPDS-a and MPDS-f combined with DCB.

Cytotoxicity of 100 μM DCB and MPDS combined with DCB on human corneal epithelial (HCE) cells. HCE cells were unaffected by incubation with 100 μM DCB for 30 min. Most MPDS with DCB showed no apparent cytotoxicity against HCE cells, except for the combinations of DCB with MPDS-a and f. Data are presented as mean±SEM from 3 independent experiments. **The means are significantly different at P<0.01 by student t-test. 120
As detailed composition of the MPDS are confidential, it is difficult to explain the mechanism that triggered the enhanced cytotoxicity upon addition of DCB. Despite the presence of cytotoxicity, it can be assumed that DCB will be diluted with tears within minutes, thereby reducing its concentration and ultimately its cytotoxicity. Furthermore, it is unlikely that cellulose biosynthesis inhibitor such as DCB will have significant effect on human cells, which lack cellulose synthesis mechanism. Our results suggested the possibility of DCB as a disinfecting agent to improve the amoebicidal activity of MPDS against Acanthamoeba immature cyst. This may be helpful in the prevention of Acanthamoeba infection associated with contact lens usage.
Supplementary materials
A most probable number of Acanthamoeba 48 hr-induced cysts after incubation with 8 commercial MPDS (solution a–h) and the combination of MPDS with 100 μM DCB. (A) showed amoebicidal activity of MPDS to 48 hr-induced cysts, and (B) showed that of MPDS combined with 100 μM DCB. Data are presented as mean±SEM from three independent experiments.
ACKNOWLEDGMENT
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education (No. 2015R1D1A1A01057265).
Notes
CONFLICT OF INTEREST
These authors have no conflict of interest related with this study.