Effect of Temperature on the Killing of Opisthorchis viverrini Eggs In Vitro
Article information
Abstract
Contaminated liver fluke egg in the environment has led to the high prevalence of human opisthorchiasis associated with cholangiocarcinoma in Southeast Asia. To find the effective lessening methods of Opisthorchis viverrini eggs in the contaminated environment, we investigated the temperature conditions for killing of these trematode eggs in vitro. Numerous O. viverrini eggs were obtained in the proximal part of uteri of adult worms from experimental hamsters. Mature eggs with miracidium were allocated by experimental groups (2 control: positive and negative and 4 treatment: 50, 60, 70, and 80°C) with 0.85% saline, and treated by the experimental plan. Eggs in each experimental groups were observed under the confocal microscope after stain with Propidium Iodide (PI) to evaluate the effect of temperatures. Eggs in 70 and 80°C groups were all killed after over 10 min heated. Majority of eggs in 60°C (10, 15, and 30 min heated), 70 and 80°C (5 min heated) groups were inactivated. However in 50°C group, below half of eggs were to be killed in all time lapse (10, 15 and 30 min). In order to prevent O. viverrini infection and cholangiocarcinoma, direct treatment of sewage by heating at 70 or 80°C at least 10 min is essential. Therefore, treatment of O. viverrini eggs at a high temperature is a potential method for controlling egg contamination in sewage.
It has been estimated that more than 40 million people are infected with foodborne trematodes [1]. In Thailand, the highest prevalence of liver fluke, Opisthorchis viverrini, is found in the northeastern region, associated with a high incidence of cholangiocarcinoma (CCA) [2]. Opisthorchiasis, O. viverrini infection, is a major public health problem in Southeast Asian countries including Laos, Cambodia, Vietnam, and Thailand [3–6]. In case of Thailand, it has been estimated that about 9 million peoples are to be infected with this endemic liver fluke. Especially, the prevalences (egg positive rates) were recorded to be 19.3% and 15.7% in residents of the north and northeastern regions respectively [7,8]. The annual economic loss due to opisthorchiasis and CCA is estimated about 120 million US dollars for medical cost and loss of labor in Thailand [9].
The eggs of O. viverrini can contaminate the environment as a result of lack of hygienic defecating habits or the use of human feces for fertilizer (night soil) [10]. One of the risk factors in opisthorchiasis is egg contaminations by reservoir hosts such as dogs, cats and wild animals as well as humans in the ecosystem of O. viverrini. Numerous studies on the infection status with this liver fluke have been reported in cats and dogs from Lao PDR and Thailand. The prevalences (egg positive rates) in fecal samples of dogs in Lao PDR were recorded 20–36% [11–13], and those of dogs and cats from Thailand were 3.9% and 36.4% respectively [14]. The higher endemicity of opisthorchiasis in the definitive hosts, i.e., human, cat and dog, contribute to the persistent maintaining of life cycle of this liver fluke in endemic areas. Therefore, it is important to find effective methods for the interruption of viable eggs contamination in the environment. The photosensitized porphyrin, an environment-friendly photoactive compound, was previously used to remove of helminth eggs in wastewater, and showed 90.0% remove efficiency [15]. The natural coagulant, seed extract of Moringa oleifera, was experimentally adopted to decrease the viability of helminth eggs in irrigation water [16]. However, the effective of the temperature for the killing of Opisthorchis viverrini eggs contaminated in the environment has not been reported yet.
To control the O. viverrini, there is the need to cut it life cycle in each step; i) eating cooked fish seems to be very simple and easy, in fact it is too difficult to change the eating culture in this region [17], ii) using good diagnostic technique [18] and effective treatment of infected human and or reservoir hosts with praziquantel [19] and iii) good sewage sanitary management systems, even though this region is having the household sanitation tank, but remains a big problem of water reservoir contaminated with liver fluke egg, which evidenced the prevalence of O. viverrini infected fish. The treatment of the human fecal material to kill O. viverrini eggs might help to reduce the prevalence of this parasite. We, therefore, expected to cut the life cycle of O. viverrini by destroying its egg in the fecal specimen using a heating method that may be used to design the sanitary tank management system. The objective of the study was to investigate the effects of temperature on the viability of O. viverrini eggs, and applied to treat the fecal specimen.
Adult O. viverrini were left over from another project (ACUC-KKU-20/2559). The eggs of O. viverrini were obtained from uteri of adult worms, which were recovered from the biliary tract of hamster at 1 month after infection. Mature eggs with miracidium were collected under a stereomicroscope after incision of the proximal portion of uterus of adults. Eggs were divided into 3 groups, 2 control groups, and a treatment group. One control group was simply maintained in 0.85% saline at room temperature (negative control). The second control group consisted of eggs killed by autoclaving at 121°C (positive control). In the experimental group, batches of eggs in 0.85% saline were heated at 50, 60, 70, and 80°C for 5, 10, 15, 30 min in a thermal cycler machine respectively. Eggs from each treatment were incubated with propidium iodide (PI) (0.5 mg/ml) for 20 min at room temperature and then observed using a confocal microscope. PI is a fluorescence dye used to distinguish between viable and non-viable cells. The membranes of viable cells exclude this dye [20]. PI passes through damaged cell membranes and intercalates with nucleic acid (DNA or RNA) to stain nuclei of dead cells [20,21]. PI cannot penetrate living eggs but produces intense color in dead eggs. A separate group of eggs (positive control) was killed by autoclaving to confirm that PI could stain dead eggs. Statistical analyses were performed using SPSS version 16 software (SPSS Inc., Chicago, Illinois, USA). All data are expressed as mean±standard deviation (SD).
At 70 and 80°C, 100% of O. viverrini eggs were dead after 10 min of incubation (Figs. 1, 2). Eggs in 60 (5, 10, 15, and 30 min), 70 (5 min), and 80°C groups (5 min) groups were killed more than 50% (Figs. 1, 2). However, at 50°C heated, O. viverrini eggs were killed less than 50% after treated for 10, 15, 30 min.
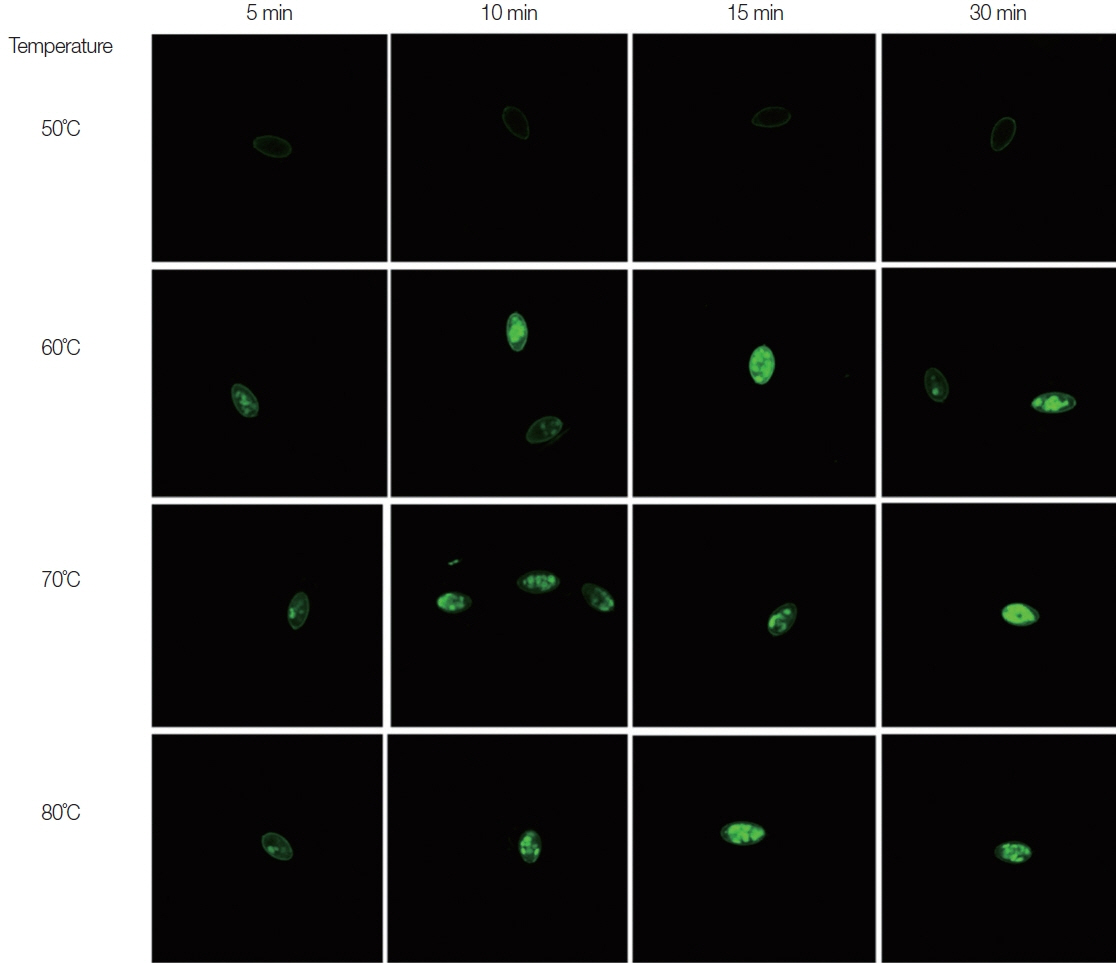
Effect of temperature on viability of Opisthorchis viverrini eggs incubated at 50, 60, 70, and 80°C for 5, 10, 15, and 30 min. Eggs in each experimental groups were observed under the confocal microscope after stain with propidium iodide. PI cannot penetrate living eggs but produces intense green color in dead eggs.
The food-borne trematode O. viverrini is the major public health problem in Southeast Asia, such as Lao PDR and Thailand [3]. Peoples get infected by eating raw or under cooked freshwater fish contaminated with metacercariae. The poor hygienic defecation into the fields that are being washed into water bodies (pond and lakes) during rainfall, causes transmission into freshwater snails [22,23]. In the northeast part of Thailand, the collection and transport service of human excreta, 78% were collected by licensed private companies, 13% of unlicensed private companies, 9% of the municipalities. Surprisingly, only 4% of all disposal sites were good practice in the excreta disposal system [23].
The disposal system of fecal sludge by private vacuum-truck into public land, grassland, or orchard and rice fields was not appropriate [24,25]. Thus, this inappropriate disposal system of untreated fecal sludge into the different site can cause the transmission of pathogens leading to human diseases [24]. In addition, 10% of the population in developing countries are infected with intestinal worm by unsafe waste and human excreta management [26].
This present study is the first report to address the effects of heating on O. viverrini eggs with sanitation management in mind. The previous study found that eggs of Echinococcus multilocularis could be killed by ultra-cold temperature (-70°C) and by rapid cooling in liquid nitrogen [27,28]. Eggs of Benedenia seriolae were killed by immersion in tap water at 50°C for 30 sec [29].
The method of fecal sludge management in Thailand are, i) treatment before releases to the environment, ii) release to the environment via the transportation without treatment, and iii) release to the environment from the toilet. Our present studies suggest that the heating of fecal sludge is one of choices for the management of pathogens (bacteria, fungi and parasites) that contaminates the environment. Direct treatment of fecal sludge by heating at 70 or 80°C at least 10 min is essential to prevent O. viverrini infection. This method could be applied in all regions, even where there is no electricity. The energy sources for boiling the sewage are solar cell, gasoline, electric currents, and natural gas from fermented agriculture waste and so on.
However, this study was limited to investigate the effect of temperature on other parasite such as Ascaris lumbricoides eggs. It well known that a shell of 3 thick layers eggs of A. lumbricoides is the most durability against different chemical and environmental factors [30]. A. lumbricoides is pathogenic parasite that has been isolated in wastewater and use for indicator for unsafe drinking-water contaminated [31–33]. Thermal techniques can reduce enteric pathogen group including bacteria, viruses, and protozoa. However, treatment process by heating method is limited in spores, which are more resistant to thermal inactivation than are vegetative cells. The conventional disinfectant for treatment of household water treatment are chlorine, chlorine dioxide, ozone, peracetic acid. Moreover, UV radiation has been used for disinfection in wastewater treatment [31,32].
In conclusion, heating at 70 and 80°C groups after 10, 15, and 30 min can kill O. viverrini eggs. This study suggests that destroying the Opisthorchis viverrini eggs in feces using heat is a possible approach for controlling egg contamination in the environment. This technique can be used for household toilet or sanitation tank management. This information may useful for implementation in endemic areas to prevent opisthorchiasis and cholangiocarcinoma in the future.
ACKNOWLEDGMENTS
This study was supported by the Cholangiocarcinoma Screening and Care Program: CASCAP (Thailand Grand Challenges: Fluke-Free Thailand) (CARI 05/2560), invitation research (IN61125) and Thailand Research Fund (RTA5580004), Faculty of Medicine, Khon Kaen University. This research was supported by the Post-Doctoral Program from Research Affairs and Graduate School, Khon Kaen University (no. 58441).
Notes
CONFLICT OF INTEREST
We have no conflict of interest related to this work.
