Survey of Flea Infestation in Dogs in Different Geographical Regions of Iran
Article information
Abstract
Medically important arthropods, including fleas, play an important role in causing clinical disorders and disease in man and domestic animals. This study was conducted to determine the seasonal flea infestations for domestic dogs from different geographic regions of Iran. A total of 407 fleas, belonging to 5 different species, were recovered from 83 domestic dogs from 3 regions. There was a distinctive pattern of species distribution and infestations with the highest infestation rates observed in a temperate climate and higher rainfall. Additionally, fleas were observed over all seasons, except February and March, with the highest infestation rate observed in August (24.7%) and the lowest rate in January (1.7%). They also parasitize dogs with a different spectrum of species. The cat flea, Ctenocephalides felis (67.5%), exhibited the highest prevalence among all flea species found on dogs. Thus, climatic conditions and seasonal patterns impact on flea infestation and must be considered in developing control programs.
INTRODUCTION
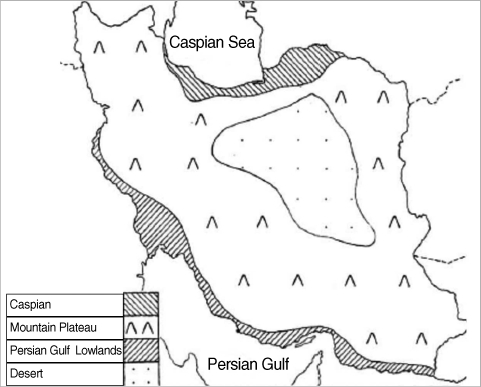
Fleas play an important role in causing clinical skin disorders and diseases in man and domestic animals [1]. Fleas are one of the most important ectoparasites with more than 2,000 species worldwide affecting mammals, birds, and reptiles [2]. In some locations, fleas represent over 50% of all the dermatological cases presented to small animal clinics. Most are limited to hosts with nests as this can provide conditions for the completion of their life cycle [3]. While fleas on pets are generally considered a nuisance that may cause some dermatologic problems they are also responsible for the transmission of several important diseases in humans and animals [4]. They have been involved in transmission of cat scratch disease (Bartonella henselae) [5,6], Rickettsia typhi (murine thyphus), Rickettsia felis [1,7-10], and also serve as the intermediate host for the tapeworm Dipylidium caninum [1] and several trypanosomatids [11]. Surveys of flea species found on dogs have recently been conducted, and it has shown that there are differences in the spectrum of flea species related to geographical areas. Several studies have been published regarding the distribution and prevalence of fleas on dogs from different parts of the world [2]. Despite the importance of the distribution of flea species on dogs and the factors affecting their distribution, no investigation of this nature has previously been conducted in different regions of Iran. Geographically, there are 4 different zones in Iran, namely Caspian Sea (region 1), Mountainous area (region 2), Persian Gulf (region 3), and the Central Desert (region 4) (Fig. 1).
The purpose of this study was to determine the pattern of flea species distribution for 3 different areas according to the method of Scherman et al. [12], including region 1 (temperature: 8-26℃, annual rainfall: 400-1,500 mm), region 2 (temperature: -5-29℃, annual rainfall: 200-500 mm), and region 3 (temperature: 12.6-35℃, annual rainfall: 200-300 mm). However, the desert area with very harsh climate conditions was excluded from this study due to extremely low animal population. This study was conducted to determine the relative flea infestation rates for domestic dogs at local veterinary clinics from September 2004 to January 2009.
MATERIALS AND METHODS
Fleas were collected from 756 dogs (279 females and 477 males) of different ages and sex from the Caspian Sea region (354), Mountainous area (331), and Persian Gulf area (71) regions of Iran. A total of 280 (37.04%) dogs of ≤ 6 months of age, 391 (51.7%) > 6 months to ≤ 2 years and 70 (9.3%) > 2 years old were sampled.
Each dog was thoroughly examined visually, going through all areas of the body, in order to establish the presence or absence of fleas. Then, each animal was combed using a 24-teeth (each tooth 2 cm in length) plastic comb [13] for 5-10 min. After combing, the flea comb was held over a white tray and the fleas collected with a forcep from the tray. From each positive dog, fleas (1-10 in number) [14] were placed in small plastic tubes containing 70% alcohol until identified. Fleas were sexed and indentified to species using routine taxonomic case keys [15-18].
The association between sex and age groups of the dogs, species of fleas, and flea infestations in different geographical areas was analyzed using the χ2-test (P ≤ 0.05).
RESULTS
A total of 407 fleas belonging to 5 different species were recovered from 83 domestic dogs in 3 regions (Table 1). Ctenocephalides felis (42.0%, n = 171) was the most frequently recovered flea species followed by Pulex irritans (26.5%, n = 108), Ctenocephalides canis (16.5%, n = 67), Xenopsylla cheopis (11.3%, n = 46) and Cediopsylla simplex (3.7%, n = 15). Female was the predominant sex collected for all species of fleas (Tables 1, 2). Among the 83 infested dogs, 56 (67.5%), 10 (12.1%), 7 (8.4%), 4 (4.8%), and 2 (2.4%) were infested with C. felis, C. canis, P. irritans, X. cheopis, and C. simplex, respectively. In addition, 4 (4.8 %) dogs had mixed flea infestations (3 dogs, C. felis and P. irritans; 1 dog, C. canis and C. felis) (Table 3). Seasonally, the fleas were collected mostly during the mid-spring and summer (Table 4). Significant differences were found in flea infestation of the dogs in different seasons (χ2 = 97.9, P < 0.05). The highest and the lowest flea infestations were found in summer and winter, respectively.
Flea infestations for regions 1, 2, and 3 were 61.5%, 36.1% and 2.4%, respectively (Table 3). Comparison of the 3 regions showed that the flea infestation in region 1 was significantly higher than region 2 (χ2 = 4.7, P = 0.03). There was no significant difference in the flea infestation between region 2 and region 3 (χ2 = 3.1, P = 0.054). The Flea infestation in region 1 was significantly higher than in region 3 (χ2 = 7.28, P = 0.005).
Flea infestations were more common in younger dogs, < 2 years (52, 62.7%), than older dogs, 2-4 years (29, 35.0%) and > 4 years (2, 2.4%). In statistical analysis using χ2-tests, significant differences were found in flea infestation of different age groups of the dogs (P < 0.05). The dogs younger than 2 years old had a higher infestation rate and dogs older than 4 years old had a lower infestation rate. Out of the 477 male and 279 female dogs examined, 45 (9.4%) and 38 (13.6%) were infested, respectively.
DISCUSSION
In our study, flea infestations on dogs were observed throughout the entire year except February and March. The highest rate of infestations was found in summer, autumn, and spring, whereas winter showed the lowest rate. The peak of flea infestation rate was 24.7% observed in August and the lowest rate of infestation stood at 1.7% in January. Results of this study are in agreement with other authors who observed that peak flea infestations occurred during the annual warm seasons [19,20].
Similar to other observations, C. felis was reported from several studies conducted in Germany [21,22], the United Kingdom [23-25], Spain [26], and in the USA [20,27] as the most common flea from region 1 but not region 2 and 3. P. irritans, the human flea, the second most common species, is similar to recent investigations from France and Germany where hundreds of human fleas were collected from dogs of single households [28].
In our study, female fleas were more abundant than males among the 5 species, which is in agreement with previous findings [20,24,26,29]. Higher female survival rates in both mature and immature stages, and/or the greater ability of females to evade capture during host grooming have been suggested as a reason for this disparity [30].
Host-dependent factors, including the sex and age of dogs, have been associated with flea distribution, with greater infestations recorded for young animals [31,32] and male dogs [33]. However, in our study, no relationship was detected between sex and flea abundance, which is similar to an earlier study [26].
Temperature and humidity are the 2 most important factors influencing survival, development, and reproduction of fleas [34]. The statistical results showed that there was a significant difference (P < 0.05) between the 3 regions with different climatic conditions and prevalence of flea infestations. Comparison of the regions indicates that as the rainfall decreases and temperature increases, flea populations decrease dramatically. The prevalence of flea infestations might also be related to the animal and human populations of the related regions with a decreasing numbers from region 1 to 3. The diversity of flea species varied regionally with 5 species in region 2, 2 species in region 1, and 1 species in region 3. Multiple infestations with > 1 flea species were uncommon and similar to the findings of the previous surveys [26,27,35].
Given that some flea-borne diseases are endemic in Iran, the results of this study can be of significant importance. For example, Kurdistan Province located in region 2 is potentially the most important focal region for plague in Iran [36]. Xenopsylla and Ctenocephalides spp. found in this region, can serve as vectors for Yersinia. Thus, flea control may constitute a valuable measure in tackling plague in this area. Murine typhus and endemic typhus fever are also particularly endemic along the coasts of the Caspian Sea in the north and the Persian Gulf in the south [36]. In another study, Dipylidium caninum has been recovered from 38.6% of mostly stray dogs in western part of Iran (Azarbaijan, Kurdestan and Kermanshah Provinces) which all fall in region 2 of the current study [37]. Cutaneous and visceral leishmaniasis exist as endemic in many parts of Iran [38-42] and there is a report of potential role for dog fleas in the cycle of Leishmania [43], though its significance still remains uncertain [44].
The significance of these findings indicate that there are geographical differences in the distribution of flea species and infestation rates, which may impact on the potential for transmission of flea-borne pathogens and dermatological diseases.
ACKNOWLEDGEMENTS
The authors would like to thank Faculty of Veterinary Medicine (Urmia University) for funding this study. The technical assistance of Mr. E. Aghapour is appreciated.