Malaria Endemicity in the Rural Communities of Ebonyi State, Nigeria
Article information
Abstract
Malaria remains a global health threat. Approximately 97% of the population is at risk in sub-Saharan countries, particularly Nigeria. This study compared the performance of 2 diagnostic methods in assessing malaria endemicity in the rural communities of Ebonyi State, Nigeria. A total of 1,140 study participants were screened for malaria parasite using Rapid Diagnostic Test kits (RDT) in the field, while thick and thin films for microscopy were examined in the laboratory. Our result showed that malaria prevalence was 56.8 by RDT and 38.6% by microscopic test. Age group under 10 years had the highest prevalence of 28.9% (RDT) and 23.6% (microscopy), respectively. The highest prevalence of 19.5% by RDT was recorded in Onicha Local Government Area, while the highest prevalence of 13.4% with microscopy was recorded in Ezza North Local Government Area. The sensitivity and specificity of microscopic examination were both 100%, while those of RDT were 95.5% and 75.9%, respectively.
INTRODUCTION
Malaria caused by infection with Plasmodium species is transmitted by mosquito-biting. The disease has a great impact on human health [1]. Malaria affects 3.3 billion people worldwide in at least 106 countries [2], but is both preventable and curable. In 2019 alone, 229 million malaria cases was detected globally with 409,000 estimated deaths (3). Children under 5 years are particularly susceptible to malaria [2], which accounted for 67% (274,000) of all malaria deaths worldwide [3].
Malaria is a serious threat to human health, repeated infections from childhood place a burden on individual households, health service, and economic development of communities and countries [4]. Malaria is one of the most important mosquito-borne diseases in Nigeria. It is more transmitted in rural communities, where favorable breeding habitats, especially during the wet season, and human activities are readily available. Malaria is a risk for 97% of Nigeria population [2] and contributes to about 11% of maternal mortality [5].
The impact of malaria is particularly pronounced in rural areas where the disease is common during periods of greatest need for agricultural labour [6]. Malaria transmission and severity of the disease vary greatly from region to region, village to village and even from person to person [7].
Factors contributing to the spread and transmission of malaria depend on the interaction between humans, Anopheles vectors, parasite, and environmental conditions. Natural transmission of malaria infection occurs by the bites of infected female Anopheles mosquitoes, but transmission can also occur by transfusion of infected blood. Infants may become infected perinatally by maternal blood.
MATERIALS AND METHODS
Ethical approval
Ethical approval was obtained from Ebonyi State Ministry of Health (Approval no. SMOH/03/017). The approval letter from the State Ministry of Health were taken to the traditional rulers as well as community leaders of the study communities for their approval of the study in their respective communities. Following approval by the community leaders, informed consent of the participants was sort through community mobilization and sensitization. The community members were invited to their town hall through the help of the community leaders, where they were explained the potential benefits of the research and allowed to consent freely.
Study areas
Ebonyi State is located in the Southeastern part of Nigeria. It shares boundaries with Benue State to the North, Enugu State to the Northeast, Abia State to Southeast and Cross River State to the East (Fig. 1). It is located between latitude 6o15’N and longitude 8o05’E. Ebonyi State is made up of 3 senatorial zones and 13 Local Government Areas (LGAs). The estimated total population in 2016 was 2,968,698 [8]. The climate of the area is tropical and the vegetation typical of the rain forest type. Average annual rainfall is 1,300 mm and average monthly temperature is of 30±5°C. There are 2 distinct seasons, the wet (between April and October) and the dry seasons (from November to March). Most rural communities in Ebonyi State is mainly flat tableland with the capacity to hold water after rainfall. These topographic features usually create breeding grounds for mosquito vectors. A number of streams and rivers traverse most rural communities in Ebonyi State and constitutes the major sources of water supply to the inhabitants of the communities. The occupation of most rural residents in Ebonyi State is primarily farming, growing major crops such as yam, cassava, and rice.
Study population
A total of 1,140 study participants aged from 1–100 years, comprised of males (n=540) and females (n=600) from 3 communities drawn from 3 LGAs in the 3 senatorial zones that make up Ebonyi State (1 LGA per senatorial zone) were screened for malaria parasite. Three hundred and eighty (380) participants were screened from each community. Participants consented to the study.
Sampling size technique
The sample size for this study was calculated using the formula below [9]
Where ME=margin of error, Z=z-score for confidence level, P=prior judgment or estimated prevalence, and n=sample size.
For this study, the confidence level was set at 95% and the z-score is 1.96: Margin of error=0.025 (2.5%). P (initial estimate of prevalence) was the prevalence of 24.5% in the rural community of Ezza North LGA of Ebonyi State [10] which puts “P” at 0.245.
Inclusion criteria
The inclusion criteria for the malaria endemicity study were individuals who have resided in the study community for the past one month before the commencement of the study. They are not seriously sick at the time of the study.
Exclusion criteria
The exclusion criteria for the malaria endemicity study were visitors to the community, infants below the age of 6 months (due to protection by passive immunity), people who have taken anti-malaria drug within the last month.
Sample collection
Malaria endemicity study was carried out in 3 communities, one each from Izzi, Onicha, and Ezza North LGAs of Ebonyi State. A total of 1,140 individuals (380 from each community) were examined for the presence of malaria parasite. All age groups ranging from 1–100 years comprised males and females were included. Sample collection was done on weekly basis. Rapid diagnostic test kits was used for the rapid diagnosis in the field while thick and thin smear slides for microscopy were prepared and transferred to the Medical Microbiology Laboratory of Ebonyi State University, Abakaliki where the slide were stained with 10% Giemsa and examined under a light microscope using x100 objective. All the samples collected each day were processed immediately after field collections. Slides that could not be examined the same day were continued the next day after proper staining. Prevalence rate was determined by calculating the percentage number of individuals infected with malaria parasite in the study population.
Rapid diagnosis
Rapid diagnosis was done using Rapid diagnostic test kit (RDT; SD Bioline malaria AG P.F/PAN kit, Standard Diagnostic. Seoul, Korea). The patient’s finger was cleaned and air dried. The cleaned finger was pricked with a sterile lancet and a drop of blood collected with a capillary device. The blood was dropped to the lower well (hole) on the RDT test kit. Two drops of buffer were added to the blood on the lower well. Fifteen minutes later, results were interpreted according to the manufacturer’s instruction and guidelines described by World Health Organization [11].
Preparation of thick blood film
A drop of blood was stirred in a circular motion with a corner of slide. The film was allowed to dry without fixative, after which the smear was stained with 10% Giemsa for 20 min. The slides were washed by placing in a jar containing water for 3 min [12], and were later examined under a light microscope using x100 objective.
Preparation of thin blood film
We smeared the blood with a swift and steady sweep along the surface of the slide. The film was air-dried and fixed with absolute methanol. The sample was stained with 10% Giemsa for 20 min [12] and were later examined under a light microscope using x100 objective.
Data analysis
Data was analyzed using SPSS version 20.0 (IBM, Chicago, Illinois, USA). Statistical difference in parasite prevalence among different age groups and sex was determined using Chi square.
RESULTS
Diagnostic performance of microscopic examination and RDT
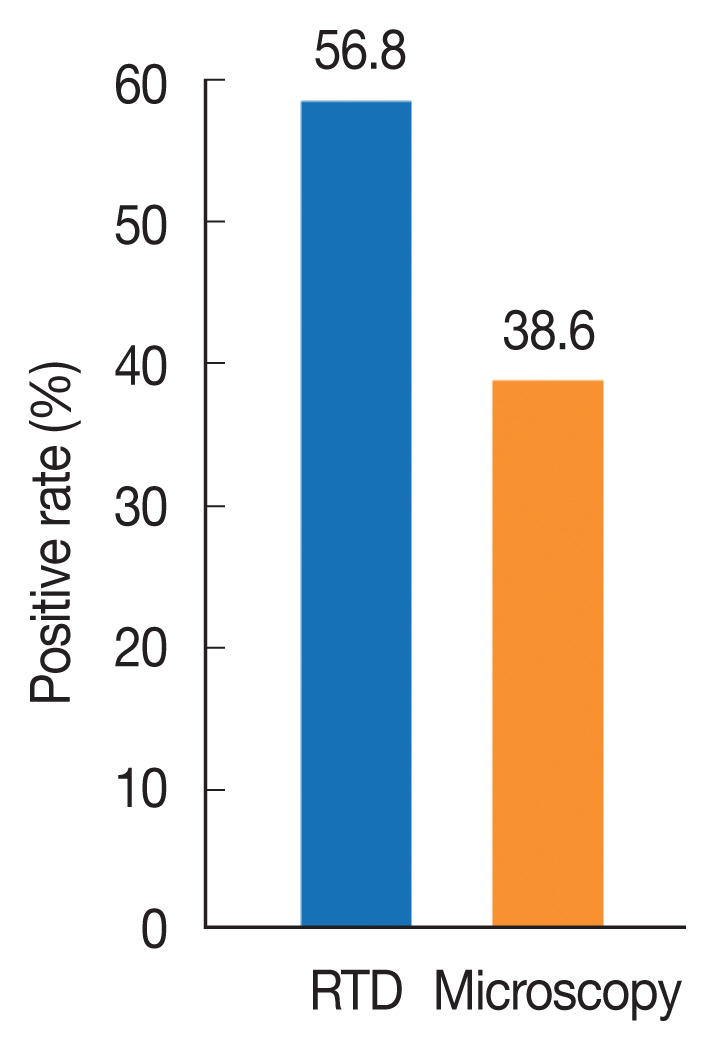
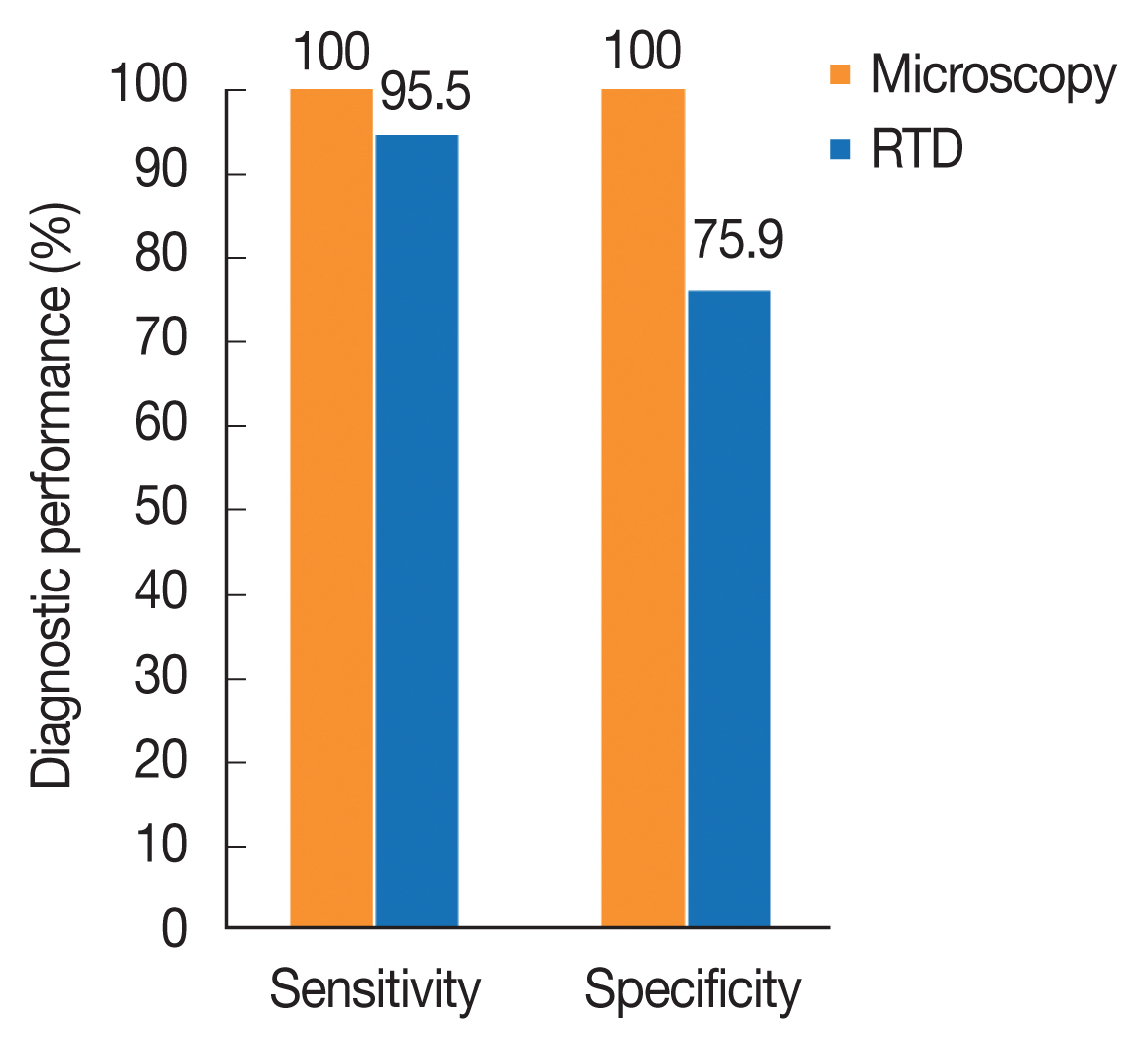
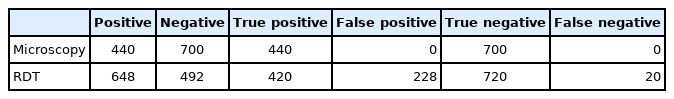
Of the 1,140 individuals examined for malaria prevalence, 648 (56.8%) showed positive with RDT while 440 (38.6%) were re-confirmed positive with microscopy (Fig. 2). There was no significant difference in parasite prevalence between the 2 diagnostic methods (χ2=0.601, df=2, P=0.741 and χ2=0.718, df=2, P=0.698), respectively. The test performance of microscopy and RDT is presented in Fig. 3. The sensitivity and specificity values for microscopic examination were both 100%. Those of RDT were 95.5% and 75.9%, respectively. All cases recorded in this study were infection with Plasmodium falciparum.
Prevalence of malaria in relation to age
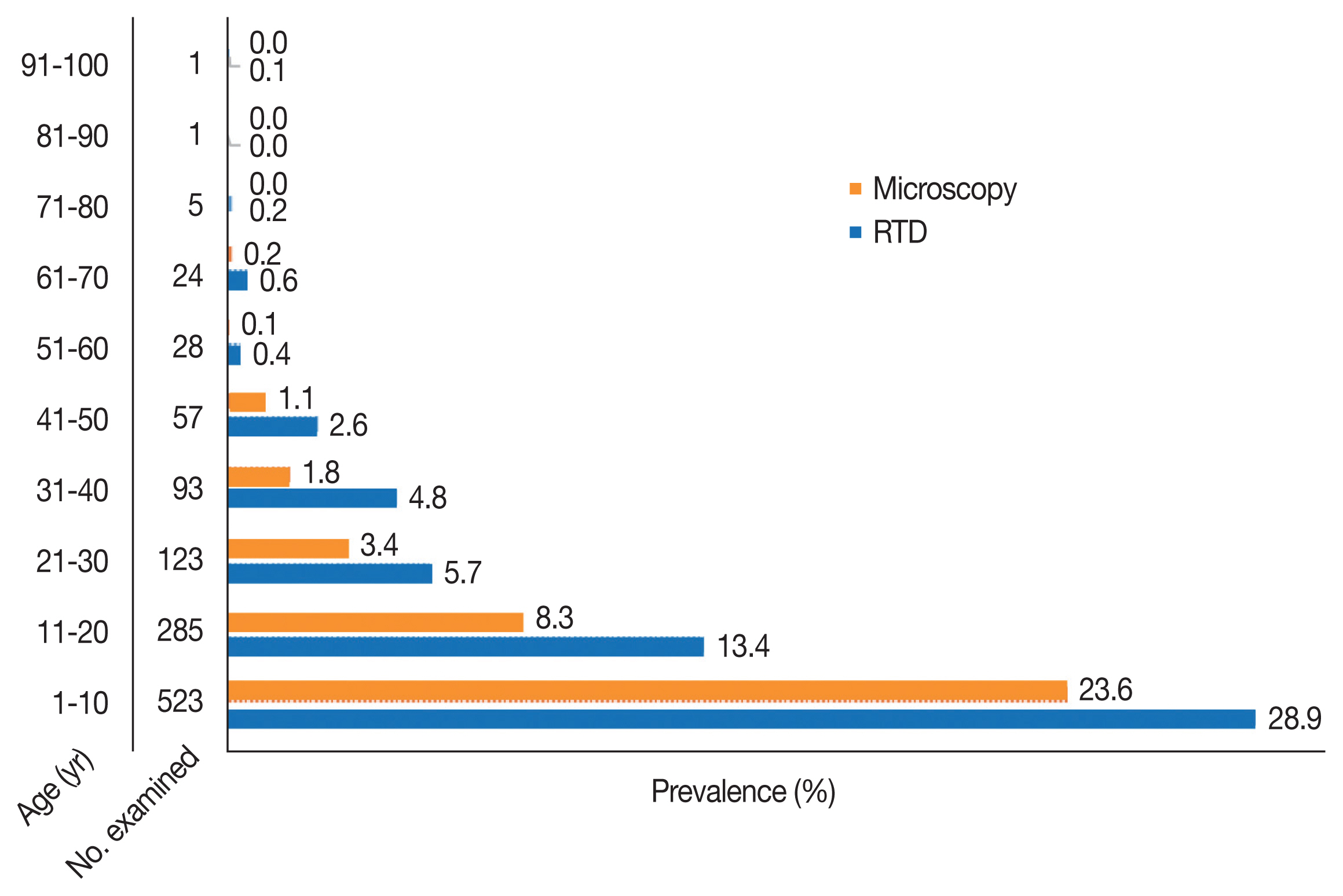
The prevalence of malaria by age group with RDT examination was highest in 1–10 years (28.9%) and lowest in 91–100 years old (0.1%). Microscopic examination showed the highest prevalence (23.6%) among 1–10 years of age and lowest (0.1%) among age groups 51–60 years (Fig. 4). Statistically, there was no significant difference in the prevalence among the age groups with both diagnostic methods (χ2=16.371, df= 16, P=0.427 and χ2=11.380, df=12, P=0.497), respectively.
Gender related malaria prevalence
The prevalence of malaria by gender is shown in Fig. 5. RDT and microscopic examination revealed the higher prevalence (31.2% and 20.4%, respectively) in males than females (25.6% and 18.2%, respectively); however, no significance difference was observed in the prevalence between males and females (χ2=0.170, df=2, P=0.919 and χ2=1.865, df=2, P=0.394).
Malaria prevalence according to locality
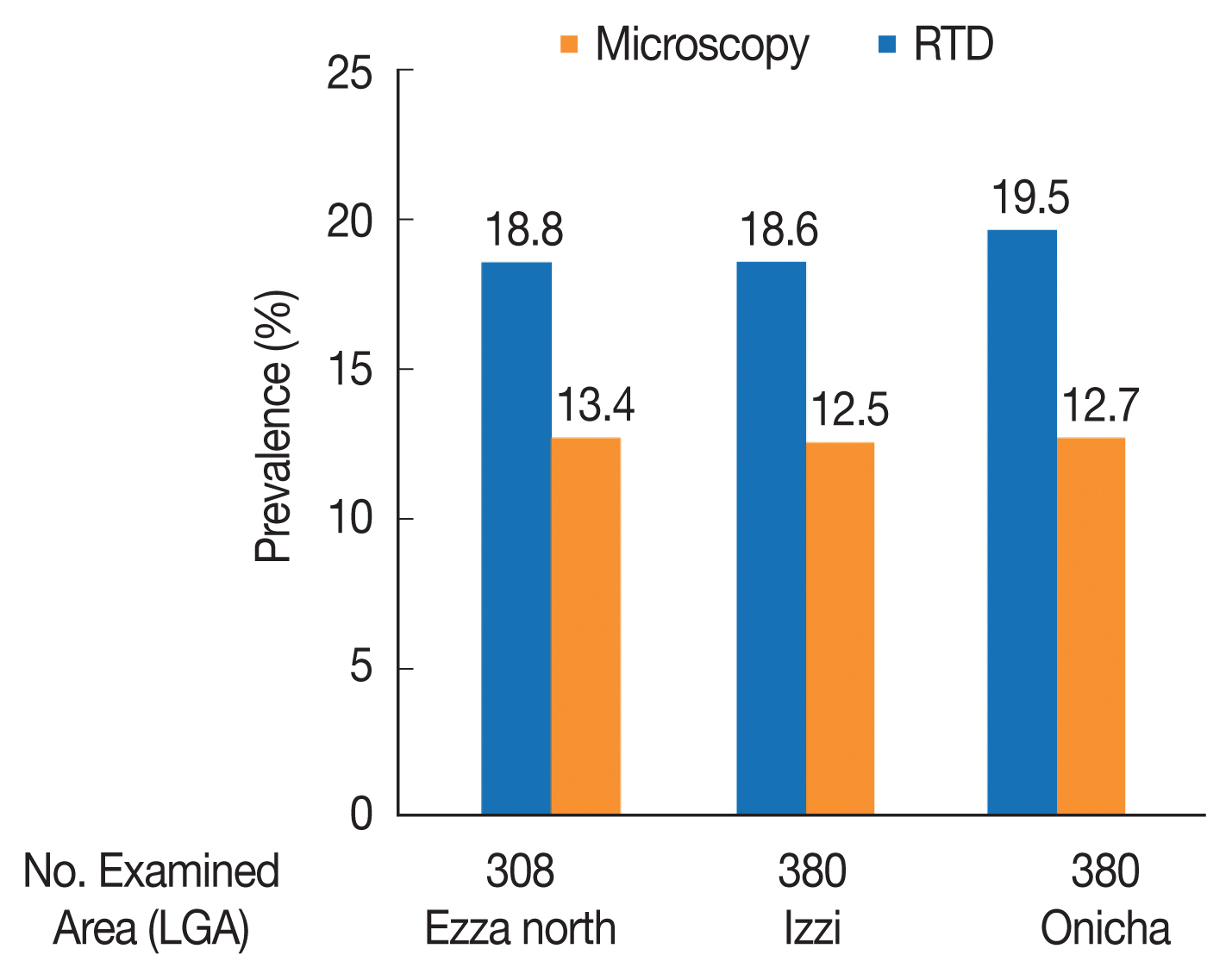
The highest prevalence of 19.5% with RDT was recorded in Onicha LGA while the lowest prevalence of 18.6% was shown in Izzi LGA. Microscopic examination showed the highest prevalence of 13.4% in Ezza North LGA and the lowest prevalence of 12.5% in Izzi LGA (Fig. 6). There were no significant differences in the prevalence of malaria among LGAs for both diagnostic methods (χ2=0.601, df=2, P=0.741 and χ2=0.718, df=2, P=0.698), respectively.
Comparison of diagnostic performance of microscopic examination and RDT
Table 1 shows a comparison of microscopic and RDT results. For microscopy, 440 samples tested were positive and 700 were negative. A total of 648 samples were positive and 492 were negative by RDT. Among the 440 positive cases diagnosed by microscopic examination, RDT failed to detect 20 cases. Out of the 700 negative cases by microscopic examination, 228 showed a positive reaction by RDT yielding 228 false positive results.
DISCUSSION
Over the past few decades, there have been great success in control of malaria. However, malaria is still prevalent in Nigeria, including Ebonyi State, where suitable climatic conditions have resulted in the wide distribution of malaria vector mosquitoes. Our results showed malaria prevalence of 56.8% with RDT and 38.6% with microscopy. This result strongly suggest that malaria is still a major public health concern in the study area. Our results are consistent with a previous report [13] indicating that malaria is endemic in Nigeria and maintains a consistently stable foci in these areas.
The malaria prevalence of 38.6% in this study by microscopic examination which is the gold standard is high. This rate is higher than previous reports, which reported that malaria prevalence was 13.7% in the West (Lagos state), 27.3% in the North (Sokoto State), 17% and 24.5% in Eastern Nigeria [10,14,15]. Other previous investigations have reported that malaria prevalence ranged from 41.7%–86.7% in Nigeria [16–18]. The malaria risk map estimated that prevalence in Nigeria varied from less than 20% to more than 70% [19].
This study showed the highest prevalence among young age group (1–10 years) by both microscopy and RDT. The result was similar to other previous reports [10,20,21], which reported a higher prevalence in young age group. There might be a slow acquisition of active immunity to malaria, since no significant difference in parasite prevalence was observed between different age groups.
Females showed a high prevalence rate. Earlier reports have also indicated high prevalence in females [10,20], while another previous study showed that male has a high prevalence rate [14], due possibly to the high risk of malaria infection by frequent exposure and cultural determinants [22]. On the other hand, other previous studies showed similar prevalence rate among the male and female in Nigeria, Kenya, and Mozambique [18,23,24]. These collective results suggest strongly that malaria risk factors are highly heterogeneous and complex [23,24].
More repeated exposure to malaria in males may lead to the development of partial immunity, which may lower risk of clinical malaria compared to females [25]. However, women’s agricultural labour, household responsibilities, such as cooking dinner outdoors, waking up before sunrise to fetch water or preparing housework, may be at greater risk of contracting malaria than men. This may explain the higher prevalence among females, as seen in this study.
It is very hard to trace the reasons for the difference in prevalence between females and males. The difference observed in this study may have been coincidental since the differences in prevalence according to the gender was not statistically significant (P>0.05). With respect to study location, there was no significant difference in the prevalence of malaria among the 3 LGAs studied. This shows that the study areas share similar characteristic that encourages vector breeding and facilitates human-mosquito vector contact.
In this study, microscopy showed higher sensitivity (100%) than RDT (95.5%). However, the reduced sensitivity of RDT is higher than the 78.4% sensitivity reported in Zaria Nigeria [26]. It is slightly higher than the 93.3% recorded by Mohammed et al. [20]. However, Brown and Azike, [27] reported very poor sensitivity of 37.7% and specificity of 89.0% in a study conducted in Port Harcourt Rivers State Nigeria. HRP-2-based malaria RDT is known for detecting malaria antigen that continues to circulate in the blood almost 2 months after the treatment of a malaria [28]. This could possibly explain the reason for the false-positive results obtained in this study.
In conclusion, malaria is still a public health problem in Ebonyi State, especially in the rural communities. The transmission appears to last most of the year due to the availability of vector breeding habitats with the consequent adult production and distribution. Accurate diagnosis of Plasmodium species is important, not only for establishing the correct treatment regime, but also for applying effective malaria control strategies in the study area.
ACKNOWLEDGEMENTS
We thank in a special way the traditional rulers of the study communities; HRH Eze Anthony Njoku (Okposi Umuoghara community), HRH Eze Ogbonnaya Ukwa (Igbeagu community) and HRH Eze Patrick Chukwu Ogudu (Onicha Igboeze community), and their town union leaders for their unalloyed support during the study in their respective communities. We equally thank Dr. Francis Onwe, the secretary Ethical Committee of the Ebonyi State Ministry of Health for his assistance in securing approval to carry out the study in the State.
Notes
The authors hereby declare that there is no conflict of interest among them.