Hypoxia-Inducible Factor-1 Alpha Stabilization in Human Macrophages during Leishmania major Infection Is Impaired by Parasite Virulence
Article information
Abstract
Hypoxia-inducible factor-1 alpha (HIF-1α) is one of the master regulators of immune and metabolic cellular functions. HIF-1α, a transcriptional factor whose activity is closely related to oxygen levels, is a target for understanding infectious disease control. Several studies have demonstrated that HIF-1α plays an important role during the infectious process, while its role in relation to parasite virulence has not been addressed. In this work, we studied the expression levels of HIF-1α and related angiogenic vascular endothelial growth factor A (VEGF-A) in human macrophages infected with promastigotes of hypo- or hyper-virulent Leishmania major human isolates. L. major parasites readily subverted host macrophage functions for their survival and induced local oxygen consumption at the site of infection. In contrast to hypo-virulent parasites that induce high HIF-1α expression levels, hyper-virulent L. major reduced HIF-1α expression in macrophages under normoxic or hypoxic conditions, and consequently impeded the expression of VEGF-A mRNA. HIF-1α may play a key role during control of disease chronicity, severity, or outcome.
INTRODUCTION
Leishmaniasis are a group of tropical diseases caused by an intracellular protozoa of the genus Leishmania. The parasite is transmitted to humans mainly by the bite of a sand fly, Phlebotomus and Lutzomyia in the Old and New World, respectively [1]. Primarily, 3 clinical forms of leishmaniasis have been reported worldwide: Cutaneous leishmaniasis (CL), visceral leishmaniasis (VL), and mucocutaneous leishmaniasis (MCL). The disease is present in 98 countries, among which CL is the most common form of leishmaniasis with an estimated 600,000 to 1 million new cases annually [1]. The disease is considered a major public health problem and belongs to the world’s most important group of tropical diseases [2]. Among the different Leishmania species, L. major is one of the causative agents of zoonotic cutaneous leishmaniasis (ZCL). In Tunisia, ZCL is the most common form with an annual incidence from 1,000 to 10,000 depending on climatic factors and the immunization of the population [3].
A major characteristic of L. major is the diversity of the disease it induces, varying from a simple self-limiting lesion to severe disfiguring lesions [4]. This clinical polymorphism might be related to the immune status of patients. Elucidating the contribution of diverse parasite intrinsic factors involved in the pathogenesis and outcome is also an important target. We previously showed that various L. major isolates differ in their experimental pathogenicity when injected into the footpad of the genetically susceptible BALB/c mice [5,6]. Understanding how intra-species parasite factors could influence disease manifestation is particularly important when the clinical severity of the lesions widely varies between patients.
When the parasite encounters the harsh environment inside host macrophage cell, a critical step is to rapidly circumvent host defenses, mainly by modulating gene expression, as shown both in murine [7] and human contexts [8–10]. Our previous study [11] highlighted the modulation, by L. major infection, of a particular microRNA, miR-210, which is transcriptionally controlled by hypoxia-inducible factor (HIF)-1α [12]. HIF-1α is a subunit of the heterodimeric transcription factor HIF-1, which was identified as a transcription factor involved in the regulation of the homeostatic response to hypoxia [13]. HIF-1α was also found to be a central regulator of inflammation, cellular innate, and metabolic functions under low oxygen levels in a wide range of diseases, from cancers to infections [14]. Previous studies have reported that HIF-1α expression was upregulated in Leishmania-infected macrophages [11,15–17]. HIF-1α silencing was hypothesized to induce infected macrophages toward a permissive Leishmania-infection status [18–20]. We showed that HIF-1α silencing led to a reduction of mirR-210 expression, as well as a concomitant decrease of parasite load in human macrophages. We hypothesized that L. major parasites induce the activation of host cell HIF-1α to counteract the natural tendency of infected macrophages to undergo apoptosis [11]. Other studies have also highlighted the stabilization of HIF-1α following Leishmania infection and its important role in the control of L. major infection [21,22]. Likewise, the angiogenic Vascular endothelial growth factor-A (VEGF-A), under the control of HIF-1α, play an important role in the control or progression of Leishmania infection. Under hypoxic conditions, cells increase the expression of genes and proteins such as VEGF that favors oxygen supply [14]. Indeed, it has been reported that following Leishmania infection, or following exposure to a state of hypoxia, the expression of VEGF-A is upregulated [23–27].
Previous studies addressing the roles of HIF-1α and VEGF-A factors during Leishmania infection have suffered from many shortcomings, such as the use of laboratory strains at late stages of culture adaptation, or clinical isolates from early cell culture adaptation showing contrasted experimental pathogenicity and virulence. This may strongly limit the identification of biomarkers with broad clinical relevance across Leishmania isolates.
The present work aims at investigating HIF-1α and VEGF-A roles as putative biomarkers by linking them to the virulence states of parasites under both normoxic and hypoxic conditions, in human macrophages infected with L. major parasites.
MATERIALS AND METHODS
Ethical statement
The study protocol, consent and ascent forms, procedures, and animal experiments were approved by the Institut Pasteur de Tunis Ethical Review Board (PV 07/07). Patients, from whom parasites where obtained, have provided written informed consent. Animal experiments were performed in compliance with the directive 86/609/EEC of the European parliament and the council on the protection of animals used for scientific purposes, in agreement with the guidelines of International Guiding Principles for Biomedical Research Involving Animals.
Parasites culture and metacyclic form purification
Two isolates of Leishmania major #0796 and #2229 were collected from 2 ZCL patients [28]. The parasites were maintained in RPMI 1640 medium (Sigma-Aldrich, Saint-Quentin Fallavier, France) supplemented with 10% inactivated fetal calf serum (Hylcone, Logan, Utah, USA), 2 mM L-glutamine, 100 U/ml penicillin and 100 U/ml Streptomycin (RPMI complete media) at 26˚C. All experiments were conducted using metacyclic purified parasites. Briefly, promastigotes were collected at the stationary phase (6–7 days of culture) and resuspended at a concentration of 1×108 parasites/ml in phosphate-buffered saline (PBS). Peanut agglutinin (PNA, 100 μg/ml) (Sigma-Aldrich) was added and the mixture was incubated for 25 min at room temperature, then centrifuged for 6 min at 150 g. The PNA negative metacyclic promastigotes present in the supernatant were washed and diluted at the desired density.
Animal infection and monitoring of the pathogenicity pattern
Female BALB/c mice were obtained from the specific pathogen-free animal-breeding facility Janvier (Gesnes, France) and maintained in a conventional animal facility. A group of 6 8-week-old BALB/c mice was infected each with 2×106 Leishmania metacyclic promastigotes. The parasites were suspended in 50 μl of PBS and injected in the left footpad of the animal. The right footpad was considered as a negative control. From the second week of infection, lesion development was assessed weekly by measuring the diameter of right and left pads by the same operator and using the same device i.e., a vernier caliper. Lesion development was then assessed by measuring the thickness diameter (mm) difference between the infected and non-infected footpad of every mouse. At the end of the experimental protocol, mice were sacrificed, and the draining lymph nodes of each infected footpad were excised and used to quantify IFN-γ, IL-4, and IL-10 mRNA cytokines. Lymph nodes from mice injected with PBS and maintained at the same conditions during the whole protocol have been used as negative controls for quantitative real-time PCR experiments.
Cell culture under normoxic and hypoxic conditions
THP-1 monocyte line was cultured in complete RPMI medium in a 5% CO2 incubator at 37˚C. Differentiation of monocytes from the human THP-1 lineage into macrophages (dTHP-1) was achieved by stimulation with Phorbol 12-myristate 13-acetate (PMA). Before infection, cells were distributed in 24-well plates for RNA extraction experiments or May-Grünwald Giemsa (MGG) coloration and in 6-well plates for proteins extraction. Cells were cultured in complete RPMI medium supplemented with 20 ng/ml PMA in a 5% CO2 incubator at 37˚C. After 24 h, the medium was changed with fresh complete RPMI, and the differentiated cells were incubated for another 24 h. DTHP-1 cells were then infected with hyper-virulent or hypo-virulent isolates of L. major with a multiplicity of infection (MOI) of 1:10, then incubated for 24 h at 37˚C. Cells incubated with CoCl2 were used as control for HIF-1α expression. Hypoxic conditions were achieved by using a GasPak EZ gas development bag system with an indicator (BD Biosciences, Le Pont de Claix Cedex, France) according to the manufacturer’s instructions.
Parasite load and infectivity
After 24 h, infected and coverslip-adherent macrophages were washed 3 times with PBS, fixed with 3.2% paraformaldehyde for 15 min, and stained with MGG. The parasite load and percentage of infectivity were determined by counting under a microscope (×40) on at least 100 cells taken randomly. The parasite load was calculated according to the formula (number of parasites/total macrophages) and infectivity ((number of infected cells/total macrophages)×100) expressed as a percentage.
RNA extraction and quantitative PCR
Mouse lymph nodes and dTHP-1 macrophages (5×105 cells) were lysed in Trizol (Qiagen, Les Ulis, France) and stored at −80˚C until extraction. Total RNA was extracted with the RNeasy mini kit (Qiagen) according to the manufacturer’s instructions. RNA concentration was determined using nanodrop ND-1000 micro-spectrophotometer. Primer sequence are as follow: for murine IFN-γ, forward, 5′-TCAAGTGGCATAGATGTGGAA-3′, and reverse, 5′-TGGCTCTGCAGGATTTTCATG-3′; for murine IL-4, forward, 5′-ACAGGAGAAGGGACGCCAT-3′, and reverse, 5′-GAAGCCCTACAGACGAGCTCA-3′; for murine IL-10, forward, 5′-GGTTGCCAAGCCTTATCGGA-3′, and reverse, 5′-ACCTGCTCCACTGCCTTGCT-3′; and for murine β2m, forward, 5′-CTGCTACGTAACACAGTTCCACCC-3′, and reverse, 5′-CATGATGCTTGATCACATGTCTCG-3′. for human HIF-1α, forward, 5′-CCATTAGAAAGCAGTTCCGC-3′, and reverse, 5′-TGGGTAGGAGATGGAGATGC-3′; for VEGF-A, forward, 5′-AGCCTTGCCTTGCTGCTCTA-3′, and reverse, 5′-GTGCTGGCCTTGGTGAGG-3′; and for human β-actin, forward, 5′-CGACAACGGCTCCGGCATGTGC-3′, and reverse, 5′-CGTCACCGGAGTCCATCACGATGC-3′. The results were normalized to those of the housekeeping gene encoding for β-actin for human samples and β2-microglobulin (β2m) for murine samples, and the results were expressed using the 2−ΔΔCt method.
Protein extraction and Western blotting analysis
Adherent cells were washed twice with PBS, scraped, and transferred to 1.5 ml tubes. After centrifugation, the cells were lysed by SDS-sample buffer at a ratio of 50 μl per 106 cells. Proteins were separated by 10% SDS-PAGE under reducing condition and transferred to a poly-vinylidène fluoride (PVDF) membrane. The membrane was blocked for 1 h with a 5% milk blocking reagent and incubated with anti-HIF-1α antibody diluted to 1:2,000 (Novus Biologicals NB100-449, Centennial, Colorado, USA) or anti-β-actin antibody (Sigma-Aldrich) diluted to 1:2,000 overnight at 4˚C. The blots were further incubated with horseradish peroxidase-conjugated host-specific antibodies for 1 h. Immunoreactive signals were detected using enhanced chemiluminescence (ECL) reagent (Amersham, Les Ulis, France) and quantification was performed by image J (https://imagej.nih.gov/ij/).
Statistical analysis
All the statistical tests were performed using PRISM software (version 5). To measure the significance of differences, we used the 2-tailed Student’s unpaired t-test. For multiple-comparison analysis, statistical significance was determined by a one-way analysis of variance (ANOVA). P values<0.05 were considered statistically significant.
RESULTS
Comparison of virulence of isolates
Two Leishmania isolates (#0796 and #2229) showing the most contrasted virulence profiles were selected based on in vivo pathogenicity and virulence in susceptible BALB/c mice, during 8-week follow-up after infection (Fig. 1A). The isolate #0796 induced a footpad lesion increase starting for 3-week after infection, whereas the isolate #2229 induced small lesion size 8-week after infection in experimentally infected BALB/c mice. At the end of the experiment (8-week post-infection), lesions induced by #0796 were almost 10 times bigger than those induced by #2229. These experimental characteristics are likely related only to the differential virulence of the isolates, although both of them were obtained during the same season from the same endemic area, and the same demographic and socioeconomic characteristics of patients from whom they were isolated.
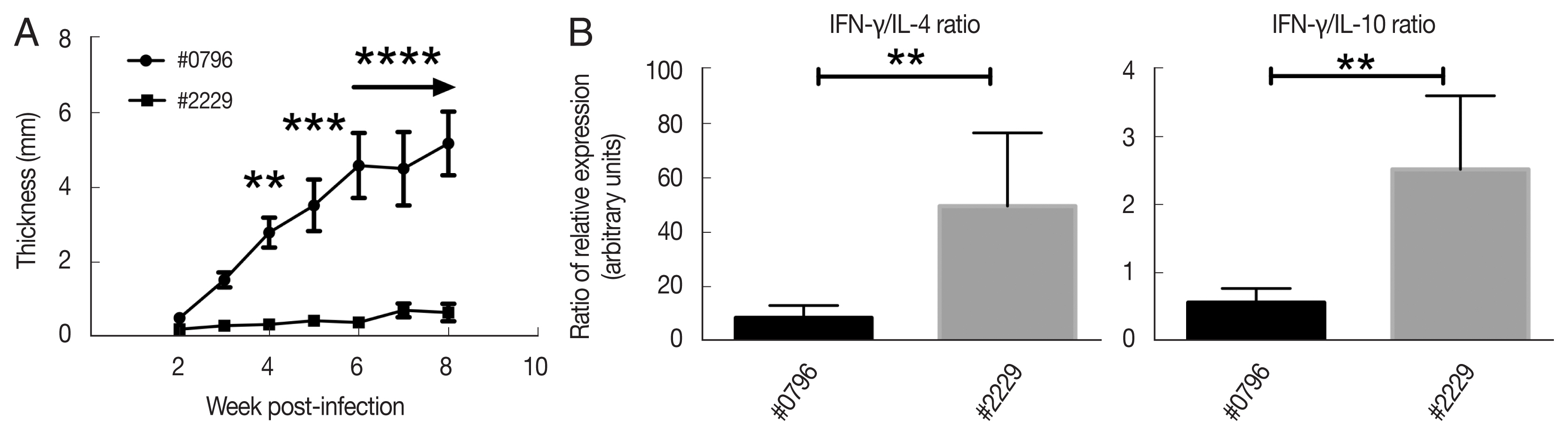
Phenotypic characteristics of isolates #0796 and #2229. (A) Lesion size of BALB/c mice footpad infected with the 2 L. major isolates. Lesion size was monitored weekly for 8 weeks. The mean and SD of each group is presented (n=6). (B) Ratios of IFN-γ/IL-4 and IFN-γ/IL-10 relative mRNA expression in draining lymph nodes of infected mice. **P<0.01, ***P<0.001, and ****P<0.0001.
Interestingly, mRNA quantification of IFN-γ, IL-4, and IL-10 mRNA cytokines in infected draining lymph node clearly showed that the IFN-γ/IL-4 and IFN-γ/IL-10 ratios were higher in the #2229 parasite isolate compared to those induced by #0796 (Fig. 1B). This indicates that infection with the Leishmania isolate #2229 induced a dominant Th1 cytokine profile compared to the profile observed with the #0796 isolate. Indeed, both ratios (IFN-γ/IL-4 and IFN-γ/IL-10) were reflecting a higher Th1/Th2 balance in #2229 isolate probably indicating a weak parasite virulence, in contrast to what was observed with #0796 isolate. Hence, our results clearly define phenotypically distinct intra-species virulent characteristics of high-virulent (HV) #0796 isolate and low-virulent (LV) #2229 isolate of lesion development and Th1/Th2 profile in infected BALB/c mice.
In vitro infectivity and parasite load under normoxic and hypoxic conditions
Hypoxia has been described to activate macrophages to control Leishmania infection [29]. To address the question of whether macrophage infections might be affected by contrasted parasite virulence under normoxic and hypoxic conditions, we assessed parasite load and infectivity rate in dTHP-1 under both conditions.
Under normoxic conditions, the HV isolate showed an infectivity rate (% of infected cells) of about 60.8%, whereas the LV isolate showed a low infectivity rate of 48.9%, 24 h after infection (Fig. 2A). When dTHP-1 were infected under hypoxic conditions, although the same trend between the 2 isolates was observed, we noticed a decrease in infectivity rates compared to those obtained under normoxic conditions. Indeed, the HV isolate induced an infection rate of 45.6% and the LV isolate induced a rate of 36.4% in hypoxic condition.
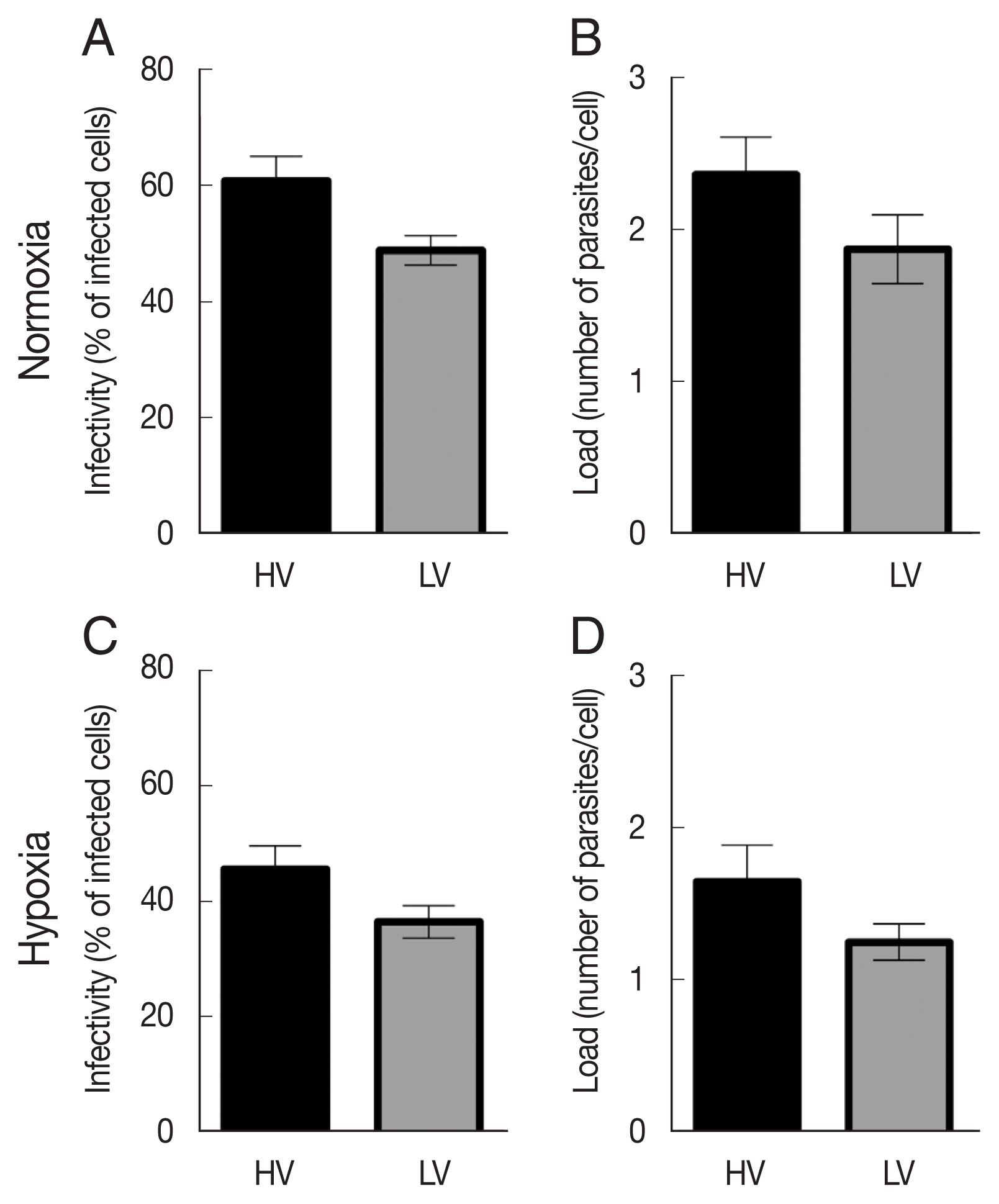
Parasite infectivity and load in dTHP-1 macrophages infected with high-virulent (HV) or low-virulent (LV) isolates for 24 h (10 parasites:1 macrophage) under normoxic or hypoxic conditions. (A) Infected dTHP-1 cells (%) after 24 h infection in normoxia. (B) Parasite load of infected cells for 24 h under normoxia. (C) Infected cells (%) after 24 h infection under hypoxia. (D) Parasite load of infected cells for 24 h under hypoxia. The data represent the mean±SEM of 4 independent experiments. Infection rates and parasite loads were carried out by 2 different operators for each experiment.
Comparable results and trends were obtained on parasite load. HV L. major isolate showed a load of 2.3 parasites/infected cell in normoxic conditions, whereas the LV isolate showed a load of 1.8 parasites/infected cell under the same conditions (Fig. 2B). We also observed a decrease of parasite loads under hypoxic conditions, where HV and LV isolates induced 1.6 and 1.3 parasites/infected cell, respectively. No changes in macrophage viability and proliferation were found in uninfected dTHP-1 cultures.
These data collectively indicate that hypoxia markedly enhance macrophage resistance to L. major infection.
Impact of L. major isolates with contrasted virulence on HIF-1α expression
We next addressed whether different parasite virulence could affect the activation and stabilization of HIF-1α in dTHP-1 cells under normal or low oxygen tensions. We analyzed the mRNA expression of HIF-1α along with time lapse via quantitative PCR experiments (0 to 24 h), either under normoxic or hypoxic conditions, in dTHP-1 cells infected by HV or LV Leishmania isolates. Fig. 3A showed that both HV and LV isolates induced a noticeable HIF-1α mRNA expression under normoxic conditions starting at early stage of infection [11].
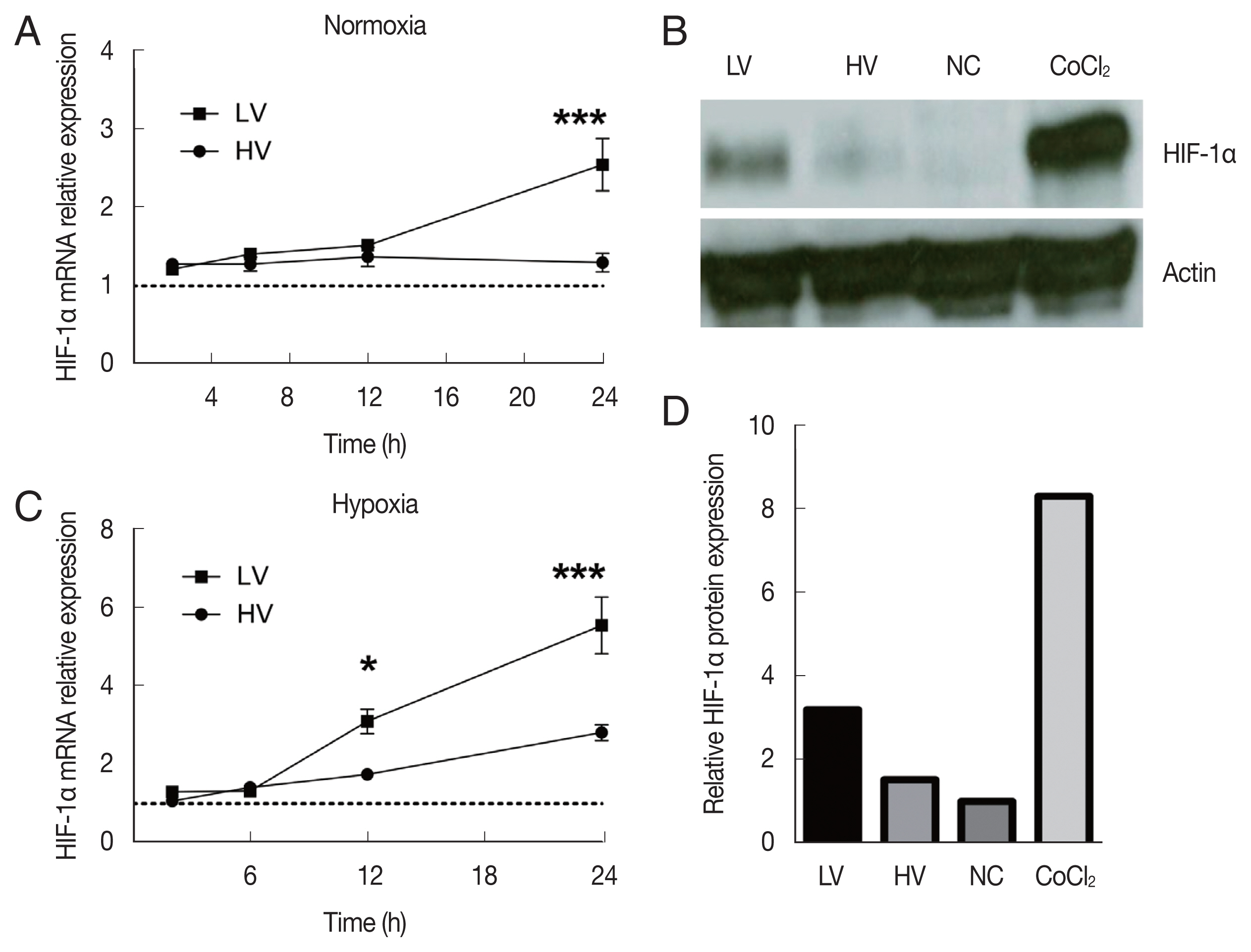
mRNA and protein expression of HIF-1α in dTHP-1 macrophages infected with high-virulent (HV) or low-virulent (LV) isolates under normoxic or hypoxic conditions. mRNA expression levels of HIF-1α in dTHP-1 were detected at 2, 6, 12, and 24 h after the infection under normoxic (A) or hypoxic (C) conditions. (B) Western blot analysis of HIF-1α protein expression levels in non-infected dTHP-1 (NC), CoCl2 stimulated cells (CoCl2), and dTHP-1 infected for 24 h with LV or HV L. major parasites under normoxic conditions. (D) Histogram showing the relative HIF-1α protein expression levels in non-infected dTHP-1 (NC), CoCl2 stimulated cells (CoCl2), and dTHP-1 infected with LV or HV parasites under normoxic conditions. The mRNA expression data (A and C) represent the mean±SEM of a triplicate representative results from 4 independent experiments *P<0.05 and ***P<0.001. The protein expression results (B and D) are representative of one experiment out of three.
Under hypoxic conditions, an enhanced expression of HIF-1α mRNA with both isolates compared to what observed under normoxic conditions was observed. This result emphasized the synergy of the infection and the oxygen level toward the recruitment of HIF-1α (Fig. 3C). However, the expression levels induced by LV isolate infection were at least twice higher than those induced with HV isolate and further indicated that parasite virulence greatly inhibited the expression of HIF-1α (Fig. 3B).
Interestingly, significant differences were observed between the 2 isolates at 24 h after infection under both conditions. The LV isolate induced higher HIF-1α mRNA expression than HV parasites.
We used this time point (24 h of infection) during the following experiments. Such expression at 24 h was almost 2 times higher in normoxic conditions when cells were infected with LV isolate than with HV isolate. When we monitored HIF-1α protein expression by western blotting, the same trends toward less HIF-1α stabilization by highly virulent parasites are observed 24 h upon infection (Fig. 3B). HIF-1α protein expression by dTHP-1 macrophages infected with LV isolate was at least 2-fold higher than in those infected with HV isolate (Fig. 3D).
Effect of different L. major virulences on vascular remodeling induced by VEGF-A expression
The low-oxygen environment initiates expression of HIF-1α and VEGF-A in macrophages during the infection [23,30]. Given that HIF-1α mediated VEGF-A and that VEGF-A/VEGF receptor 2 (VEGFR-2) signaling induces lymph angiogenesis, we investigated the association between lymphangiogenesis and parasite virulence during L. major infection.
Under normoxic conditions, macrophages infected with LV isolate expressed about 6.5 times more VEGF-A mRNA levels than those infected with the HV isolate (Fig. 4A). dTHP-1 infection with LV parasites in the absence of oxygen induced VEGF-A mRNA expression almost identical (about 13 arbitrary relative gene expression units) to that observed under normoxic conditions (Fig. 4B). Moreover, although the expression levels were increased with HV parasites (2.1 arbitrary relative gene expression units in normoxia vs 4.8 in hypoxia), macrophages infected with the HV isolate were not able to restore the VEGF-A expression. These collective results show that infection with the LV isolate under normoxic conditions is maintaining the ability of host macrophages to induce a very high level of VEGF-A mRNA expression, and that this elevated expression appears to reach a saturating level under hypoxic conditions. On the contrary, the HV parasites are able to downregulate VEGF-A mRNA expression, a process by which they could more efficiently limit angiogenesis and immune cell recruitment.
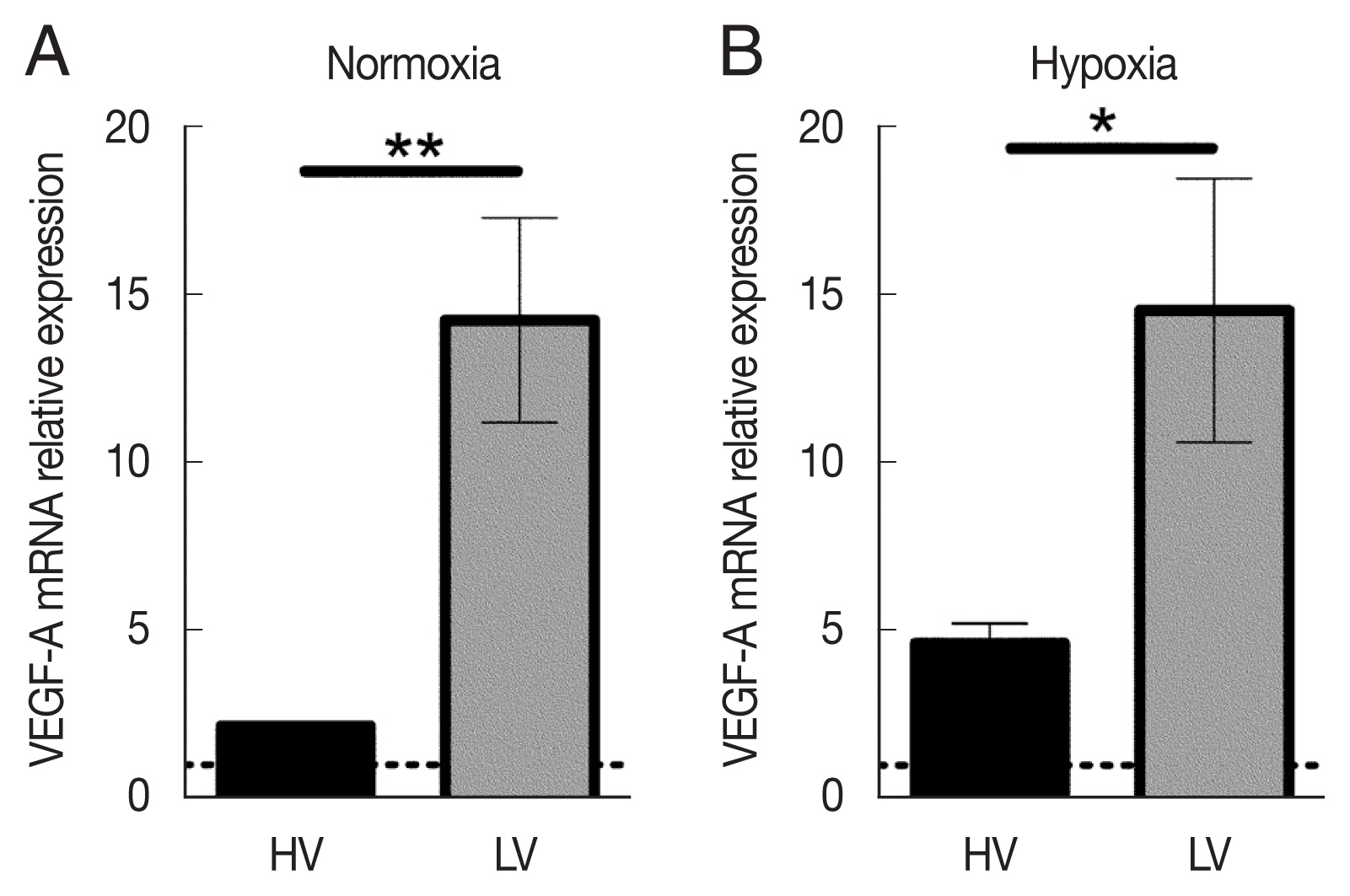
VEGF-A mRNA expression in dTHP-1 macrophages infected with high-virulent (HV) or low-virulent (LV) isolates under normoxic or hypoxic conditions. (A) mRNA expression levels of VEGF-A in dTHP-1 infected with HV or LV isolate of L. major for 24 h under normoxic conditions. (B) mRNA expression levels of VEGF-A in dTHP-1 infected with HV or LV isolate of L. major for 24 h under hypoxic conditions. The mRNA expression data represent mean±SEM of 4 independent experiments. *P<0.05 and **P<0.01.
DISCUSSION
HIF-1α is considered a regulator of cellular metabolism and innate immune cell functions. Activation of HIF-1α is a general phenomenon in infections with human pathogenic bacteria, viruses, fungi, and protozoa. Intracellular pathogens such as Leishmania have been reported to manipulate host cell metabolism to escape macrophagic killing. Indeed, Leishmania-induced hijacking of cellular metabolism of macrophages results in the decrease of host defense mechanisms and is therefore considered an important pathogen escape route.
No studies have been conducted to assess the effect of parasitic virulence on the expression of HIF-1α transcription factor in vivo or in vitro. We hence monitored the expression of HIF-1α in infected dTHP-1 macrophages using 2 well characterized L. major isolates freshly obtained from ZCL patients and show a contrasted virulence profile in the experimental model. The 2 isolates were classified as LV and HV according to the lesion size observed in infected animals 8 weeks upon infection and to the cytokine profile in lymph nodes draining the site of infection in BALB/c animals.
Interestingly, our results show that HV Leishmania parasites induce a relatively low expression levels of both mRNA and protein of HIF-1α compared to LV L. major. Recently, the early secreted antigenic target 6-kDa (ESAT-6), which is an essential virulence factor of the intra-macrophagic pathogen Mycobacterium tuberculosis, has been shown to increase the expression of HIF-1α mRNA and protein and enhanced reactive oxygen species (ROS) generation in dTHP-1 macrophages treated with soluble ESAT-6 [31]. In addition, it appears that HIF-1α worsens Klebsiella pneumoniae infection through the secretion of small iron-chelating siderophores molecules and bacterial virulence factor [32]. In contrast to these studies, our results indicate that HV L. major parasites decrease the expression of HIF-1α mRNA and protein in infected macrophages. Our results clearly contrasted to previous study, which reported that infection of murine macrophages with L. major alone was not sufficient to promote HIF-1α accumulation in vitro but required additional exogenous inflammatory signals [33]. Our data should be further confirmed whether HIF-1α inhibits parasite phagocytosis as described elsewhere [34].
Studies on the recruitment and role of HIF-1α during infection with other Leishmania species highlight the functional duality of this transcription factor. In mice infected with L. donovani, the stabilization of HIF-1α promotes disease development [35]. On the other hand, the stabilization of HIF-1α in mice infected with L. major promotes the self-resolution of the lesion [33]. Whether our observations are due to an intrinsic Leishmania virulence factor or due to the higher loading of the parasites per se is a question to be addressed.
Infection of both isolates with dTHP-1 cells under hypoxic conditions reduced parasite load and enhanced HIF-1α mRNA expression. Our results corroborate those obtained with L. amazonensis-infected macrophages under hypoxia, with a significant decrease in the proportion of infected cells and the number of parasites [36]. Either under hypoxia or normoxia, our results clearly indicate that virulent L. major parasites inhibit the expression and stabilization of HIF-1α to escape macrophage killing.
It is known that macrophages produce VEGF-A, which binds to VEGFR-2 on lymphatic endothelial cells to induce lymphangiogenesis. The production of VEGF-A is dependent on HIF-1α signaling. We found that HIF-1α activation promoted the ability of macrophages to drive lymphatic remodeling through VEGF-A mRNA expression during infection with LV promastigotes under normoxic conditions. This VEGF-A mRNA production appears to be stable under hypoxic conditions, in contrast to HIF-1α mRNA accumulation. However, macrophage infected with HV promastigotes drastically impairs VEGF-A mRNA production under hypoxia or normoxia. Expression of HIF-1α under hypoxic environment can initiate the expression of VEGF-A in murine macrophages during L. major infection [30].
In mice infected with L. amazonensis, higher levels of VEGF-A expression were detected in healed mice compared to the non-healed mice [26], which suggest that the expression levels of VEGF-A could predict the outcome of the infection [18,26]. This is consistent with our results that, under normoxic or hypoxic conditions, LV parasites with self-resolving lesions induced high levels of VEGF-A, whereas infection by HV promastigotes with persistent parasites produced low levels of VEGF.
In conclusion, the identification of parasitic factors responsible for HIF-1α and VEGF-A inhibition by macrophages holds an critical role of hypoxia-dependent parasite clearance during CL, which is the focus of ongoing investigation in our laboratory. In vitro or in experimental models, such factors are proven to play an important role in the first line of skin host defense against L. major infection. It is a problem to be addressed in future human infections.
ACKNOWLEDGMENTS
This work was supported by the Tunisian Ministry of Higher Education and Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We thank the Animal Facilities of the Institut Pasteur de Tunis for their help conducting this study.
Notes
The authors declare no conflict of interest related to this study.
