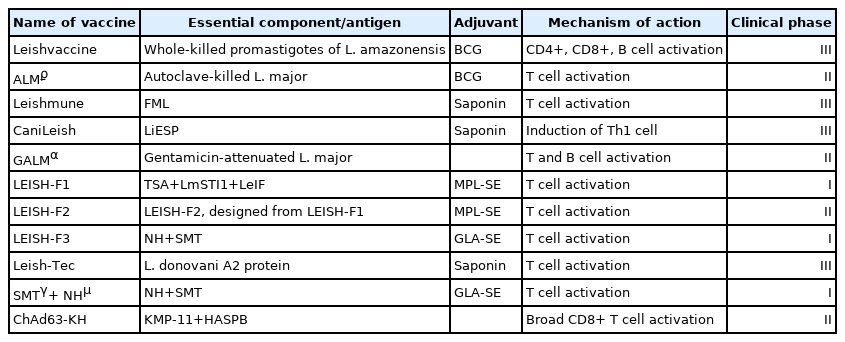
Leishmania Vaccines: the Current Situation with Its Promising Aspect for the Future
Article information
Abstract
Leishmaniasis is a serious parasitic disease caused by Leishmania spp. transmitted through sandfly bites. This disease is a major public health concern worldwide. It can occur in 3 different clinical forms: cutaneous, mucocutaneous, and visceral leishmaniasis (CL, MCL, and VL, respectively), caused by different Leishmania spp. Currently, licensed vaccines are unavailable for the treatment of human leishmaniasis. The treatment and prevention of this disease rely mainly on chemotherapeutics, which are highly toxic and have an increasing resistance problem. The development of a safe, effective, and affordable vaccine for all forms of vector-borne disease is urgently needed to block transmission of the parasite between the host and vector. Immunological mechanisms in the pathogenesis of leishmaniasis are complex. IL-12-driven Th1-type immune response plays a crucial role in host protection. The essential purpose of vaccination is to establish a protective immune response. To date, numerous vaccine studies have been conducted using live/attenuated/killed parasites, fractionated parasites, subunits, recombinant or DNA technology, delivery systems, and chimeric peptides. Most of these studies were limited to animals. In addition, standardization has not been achieved in these studies due to the differences in the virulence dynamics of the Leishmania spp. and the feasibility of the adjuvants. More studies are needed to develop a safe and effective vaccine, which is the most promising approach against Leishmania infection.
INTRODUCTION
Leishmaniasis is a serious parasitic disease caused by Leishmania species transmitted by sand fly bites [1]. The clinical manifestations can be classified as cutaneous (CL), mucocutaneous (MCL), and visceral leishmaniasis (VL). The clinical form depends on the complex interactions between the virulence characteristics of the infecting Leishmania spp. and the immune responses of the host [2]. This disease is a major public health concern. The CL form tends to heal spontaneously and often causes disfiguring scars on the skin [3]. Although a history of CL is not reported in all new MCL patients, MCL results from the spread of parasites to the oral, nasal, pharyngeal, and laryngeal mucosa following or simultaneously with CL. Spontaneous recovery from MCL is rare and can progress to severe deformities such as nasal septal destruction, airway obstruction, and eventually death [4]. The VL is the most severe form and potentially fatal if left untreated [5]. The disease has been reported as endemic in 98 countries and territories in 2020. It primarily affects poor people in Africa, Asia, and Latin America [6]. Despite being the second most common parasitic disease after malaria and its devastating nature, it has remained one of the most neglected diseases worldwide until recent years, perhaps due to its rarity in developed countries [7,8].
Currently, licensed vaccines are unavailable for the treatment of human leishmaniasis [9]. The treatment of the disease and prevention of its spread in the community is mainly dependent on chemotherapy [10–12]. Of the more than 25 compounds, pentavalent antimonials (Sb5+) are the first-line chemotherapy drugs. Amphotericin B, pentamidine isethionate, miltefosine, and paromomycin are the second-line chemotherapeutics [1,13]. However, the existing drugs are not ideal because of their high toxicity and resistance [2,12]. Although the CL form can be treated with thermotherapy, it is not feasible for the other forms of the disease [14].
According to the World Health Organization (WHO), vaccination is most likely the best method to control the disease and avoid the unwanted effects of chemotherapies [14]. The development of a safe, effective, and affordable vaccine for all forms of the disease is one of the most promising approaches and the main priority of global public health [15]. Hence, this article aims to focus on the current status and promising results of the available vaccines evaluated in different modalities.
THE ROLE OF IMMUNOLOGICAL MECHANISMS IN LEISHMANIASIS
Unlike most parasitic infections, protective immunity occurs against reinfection in patients who recover from leishmaniasis or following leishmanization (LZ) [16,17]. This result reveals that immunological mechanisms play a major role in shaping the disease, and this globally important disease can potentially be prevented using vaccines [8,18,19]. It also provides a roadmap for the development of successful vaccines that can generate protective immunity against infection [20].
The immunological protective mechanism is complicated in leishmaniasis [21]. Cell-mediated immunity plays a crucial role in host immunity. While the primary immune response to infection is initiated through the innate branch, which develops protective innate and adaptive immunity, infection control is mainly mediated by IL-12-driven Th1-type immune response. T lymphocytes shape the host immune response to provide direct protective or non-protective immunity [22]. The production of IFN-γ by CD4+ T cells activates macrophages to kill parasites under nitric oxide (NO)-mediated conditions [23]. Disease progression is largely driven by the production of non-protective IL-4-driven Th2-associated cytokines IL-4, IL-10, IL-13, and TGF-β [24–26]. Some species, such as L. mexicana and L. amazonensis in the New World, unlike L. major, can survive in conditions of limited Th1 immune responses in the host [27]. Unlike Th1, the Th2-type immune response is unable to neutralize intracellular parasites, causing the parasite to spread into VL or, for New World species, disseminated cutaneous leishmaniasis (DCL) [28].
VACCINATION STRATEGIES IN LEISHMANIASIS
The availability of vaccines against one or more forms of leishmaniasis can reduce mortality and morbidity associated with this disease. However, Leishmania parasites have a complex life cycle, which consists of stages in animal/human and sand flies and is the most important barrier for vaccine development. Moreover, this neglected tropical disease substantially affects low- and lower-middle-income countries, which discourages commercial developers from invest in the studies of vaccine [8]. In addition, differences in the virulence dynamics of Leishmania species and the immune responses induced by them, as well as the suitability of adjuvants negatively affect vaccine development and standardization efforts [7]. A vaccine against CL caused by L. major may not necessarily have effectivity against the New World forms of diseases, including MCL and DCL [29]. Nonetheless, various vaccination strategies, such as recombinant antigens, DNA vaccines, salivary gland proteins, killed parasites, and live attenuated parasites exist to treat leishmaniasis [30–34].
An ideal antileishmanial vaccine may solve the current problems and must be safe, stable, reproducible, less risky, easily administered, stored, and delivered, not reversible to an infectious state, and able to induce long-term immunological memory [8,35]. However, no vaccine meets these ideal specifications [36]. Compared with other approaches, the strategy of using live vaccines is more attractive because of the induction of a response similar to the immunological response in the natural course of infection (Fig. 1). In the live vaccine strategy, the entire spectrum of antigens is presented to the host immune system without an adjuvant [21].
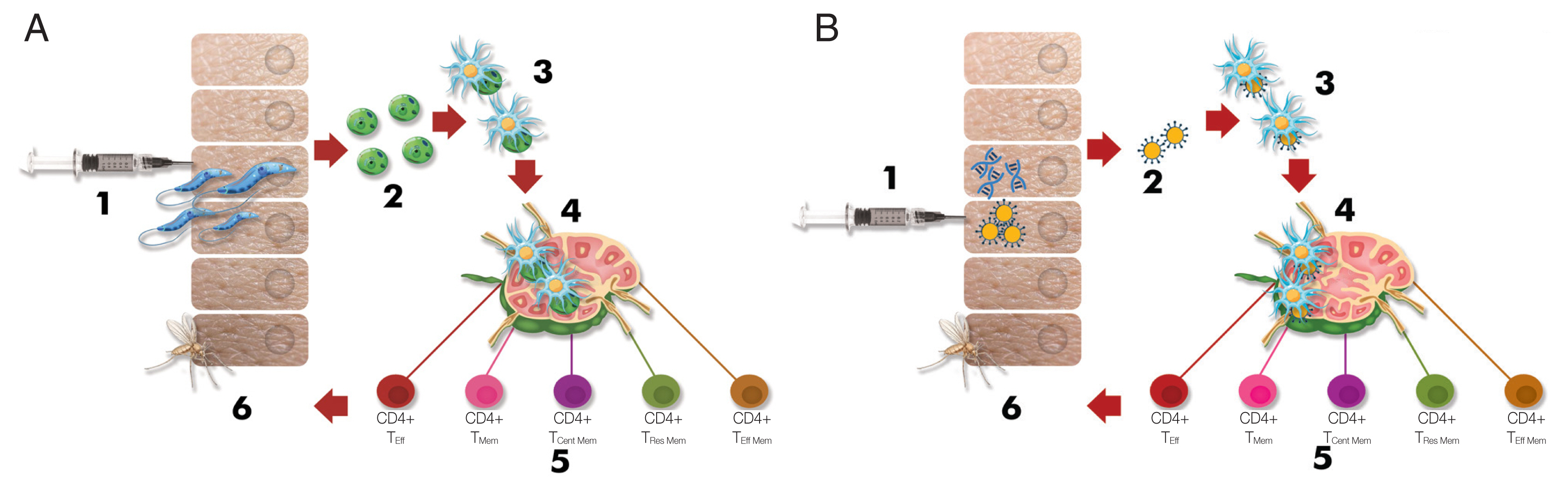
Schematic depiction briefing the immunological response against Leishmania infection (Modified from Pacheco-Fernandez et al. [36], with permission). (A) Immunological response in live/attenuated Leishmania vaccination. 1) Injection of live/attenuated parasites, 2) Transforming promastigotes into amastigotes, 3) Internalization of the amastigotes by dendritic cells, 4) Presentation to T cells in the draining lymph nodes by dendritic cells, 5) Differentiation of T cells into effector and memory T cells, 6) Prevention of transmission to the sand fly because of long-term protective immunity. (B) Immunological response in Leishmania vaccination using DNA, recombinant antigen (Ag), or subunit Ag. 1) Injection of DNA, recombinant Ag, or subunit Ag, 2) Encountering of antigens and dendritic cells, 3) Internalization of the antigens by dendritic cells, 4) Presentation to T cells in the draining lymph nodes by dendritic cells, 5) Differentiation T cells into the effector and memory T cells, 6) Prevention of transmission to the sand fly because of long-term protective immunity. CD4+ TEff: effector T helper cell, CD4+ TMem: memory T helper cell, CD4+ TCent Mem: central memory T helper cell, Tissue TRes Mem: tissue residence memory T cell, effector T helper cell, and CD8+ TEff: effector cytotoxic T cell.
Potential interventions to induce an immune response against Leishmania can be analyzed using different strategies, such as LZ and saliva vaccines, as well as first-, second-, and third-generation vaccines [28]. Different novel approaches have been investigated for this purpose, such as delivery systems and chimeric peptides. These approaches can be designed as single or cocktail antigens [36–38].
Leishmanization (LZ) vaccine
LZ is an ancient practice of vaccination [28]. LZ is an intradermal low-dose inoculation of live and virulent L. major leading to a single lesion [39]. LZ provided greater than 90% protection against reinfection [16]. LZ has been used in several countries in the Middle East and the former Soviet Union. Except for Uzbekistan, which is an endemic country, it is no longer practiced due to safety concerns such as HIV spread, use of immunosuppressive drugs, ethical reasons, uncontrollable persistent skin lesions, and persistence of parasites [16,21]. The overall strategy should be to develop a safer vaccine by providing protective immunity without causing skin lesions, including in immunosuppressed individuals. However, vaccination with live parasites showed a stronger Th1 type of immune response than vaccination with killed parasites, which exhibited limited protection. However, the reason for these differences is not well known [17].
Another study from Iran showed that leishmanization was found to reduce the incidence of the disease between 1/6 and 1/8 of its original level in a hyper-endemic region of Iran and thus was recommended for people at high risk of contracting the disease [39].
Saliva vaccine
Sand fly vectors (Phlebotomus and Lutzomyia spp.) are of particular interest and induce immune responses as adjuvants through certain immunogenic proteins such as LJM19 and LJL143 from L. longipalpis and PdSP15 from P. duboscqui [16,40,41]. Normally, sand fly saliva is known to enhance the infection caused by Leishmania spp. [41]. However, pre-exposure to saliva protected mice against parasitic infections. In a study on mice, Carregaro et al. [18] reported that the injection of sand fly salivary gland extract resulted in increase in IFN-γ and IL12 production in the site of inflammation and pre-exposure to saliva protects mice against parasitic infections. They concluded that the generation of new saliva vaccine strategies may help prevent Leishmania establishment in the host.
Immunization with a vehicle expressing the vector salivary protein expressing the PpSP15 protein, such as Lactococcus lactis and Leishmania tarentolae, has been shown to protect against CL or VL [42,43].
First generation (whole-killed or fractioned parasite) vaccines
First-generation vaccines are composed of whole-killed parasites or partially purified fraction(s) or excreted from the parasite. These have been replaced with LZ [28]. The parasites were killed using different methods, such as long-term in vitro culture, temperature, pressure, γ-radiation, and chemicals [44–49]. The killed parasite vaccines present a huge repertoire of parasite antigens and can promote significant protection against infection by mimicking natural infections. However, these studies show that they induce a weaker Th1 type immune response than live parasites, and the results are associated with an inconsistent effect. For these reasons, adjuvants have been used in many studies (e.g., Bacillus Calmette–Guérin (BCG)), and the parasites have been administered through alternative routes such as the mucosal route [19,39, 49].
Whole-killed vaccines
The Leishvaccine, which comprised whole-killed promastigotes of L. amazonensis strain (IFLA/BR/1967/PH8) and BCG, plays an important role against canine leishmaniasis. The vaccine significantly increases cytokine expression, innate immunity, and adaptive immune response [50]. The leishmania vaccine was successful in clinical trials of human Phases I and II for its safety and immunogenicity; however, it failed in Phase III. The application of autoclaved-killed L. mexicana adjuvanted with BCG resulted in low levels of leishmanin skin test (LST) conversion, which is a marker for cellular immune response [51]. Nevertheless, the incidence of leishmaniasis has significantly decreased in LST-converted participants [49]. Similarly, autoclaved-killed L. major (ALM), the old-world species associated with BCG, caused LST conversion in approximately one-third of healthy participants. However, there was a significant reduction in the incidence in individuals who LST-converted [52].
In addition to their preventive aims, the use of this type of vaccine for immunotherapy offers a safe option for severe forms of CL that do not respond to conventional chemotherapy. In a multicenter randomized controlled clinical trial (RCT) that evaluated the effects of immunotherapy with a vaccine comprising heat-killed L. mexicana+L. amazonensis adjuvanted with BCG over 10 years, 95.7% of patients with CL were treated with mild adverse events and low cost [49]. The immunotherapeutic approach has been successful in cases of mucocutaneous and diffuse forms of CL [52]. Furthermore, a combination of alum-precipitated ALM (alum/ALM)+BCG and sodium stibogluconate (Stb) was shown to be more effective than Stb alone (87% vs. 53%) [53].
Fractionated Leishmania antigens
Four fractionated vaccines, Leishmune (Zoetis Industria de Produtos Veterinarios LTDA, São Paulo, Brazil), Leish-Tec (Hertape Calier Saúde Animal S/A, Juatuba, Brazil), CaniLeish (Virbac, Carros, France), and LetiFend (3P Biopharmaceuticals SL, Navarra, Spain), have been licensed and have achieved impressive success in preventing canine leishmaniasis. Of these, Leishmune and Leish-Tec in Brazil, and CaniLeish and LetiFend in Europe have been commercialized [54].
Leishmune is composed of the fucose-mannose ligand (FML) of L. donovani and saponin adjuvant [55]. In phase III studies conducted in dogs, 92% protection against disease was observed. Because no clinical signs or Leishmania DNA were detected in these animals, Leishmune was evaluated as a transmission-blocking vaccine. However, the lack of sample randomization or blinded evaluation of trial individuals did not allow for full validation of the results. In 2014, the production and marketing license of Leishmune was withdrawn [54]. The CaniLeish vaccine is composed of purified excreted–secreted proteins of L. infantum (LiESP) and adjuvanted with saponin (named QA-21) [56]. The vaccine elicited predominantly Th1-type cellular immune responses, and the infection protection rate was 99.4% in a field study [57].
Leish-Tec and LetiFend are recombinant vaccines. Leish-Tec was formulated with recombinant protein A2 from L. donovani amastigotes and saponin as a vaccine adjuvant. In studies that assessed Leish-Tec, it was observed that it was effective not only as preventive, but also in immunotherapeutic approaches [54]. LetiFend contains a chimeric protein (protein Q) formed by 5 antigenic fragments from 4 different L. infantum proteins (ribosomal proteins LiP2a, LiP2b, and LiP0, and histone H2A), to which no adjuvant has been added. It was shown it would be the potential of protein Q in the immunization against L. infantum in preliminary studies in mice [58].
Fractionated Leishmania vaccines seem to be efficiently used in areas that are crucial to the control of Leishmania infection [56].
Second generation (subunit or genetically modified parasite) vaccines
Although first-generation vaccines are still being evaluated, several studies have focused on second-generation vaccines. Second-generation vaccines include different recombinant proteins, which are produced through genetically engineered cells such as viruses and bacteria, purified native protein fractions of parasite antigens, synthetic peptides, and even genetically modified parasites [28]. Second-generation vaccines are more feasible for mass vaccination, and their recombinant nature facilitates accessibility to large-scale and cost-effective production [54].
Subunit vaccines
The several subunits or recombinant vaccine candidates such as LeIF, gp63, p36/LACK, A-2, PSA-2/gp46/M-2, FML, LCR1, ORFF, KMP11, LmSTI1, TSA, HASPB1, protein Q, cysteine protease B (CPB), and A (CPA) have been extensively studied [40,55,59–67]. Many subunit vaccine candidates stimulate an effective protective immune response in the prevention of Leishmania infection. One of the advantages of subunit vaccines is that they pose no risk of infection, which ensures their suitability for immunocompromised individuals [59].
Application of a surface-expressed glycoprotein (gp63), another subunit protein, in a cationic liposome increased the number of IFN-γ-producing effector T cells. Furthermore, vaccination based on gp63 DNA elicited immune responses and conferred protection [60]. In a couple of experiments assessing protective immunological effects, viruses expressing the LACK (Leishmania homologue for receptors of activated C kinase) antigen, with or without adjuvant, showed good protective effects against Leishmania infection [61,62]. Similarly, the virus expressing the promastigote protein surface of G46/M-2/PSA-2 protected against L. amazonensis [63].
Notably, recombinant antigen vaccines such as LEISH-F1, LIESH-F2, and LEISH-F3 have reached phase II clinical trials, demonstrating their potential as vaccine candidates against leishmaniasis [68–70]. LEISH-F1 is an artificial protein encoded by 3 genes: L. major homologue of eukaryotic thiol-specific antioxidant (TSA), L. major stress-inducible protein-1 (LmSTI1), and L. braziliensis elongation and initiation factor (LeIF). LEISH-F1 protein applications were evaluated following emulsification of monophosphoryl lipid A in a structure-stimulating toll-like receptor (MPL-SE). LEISH-F1+MPL-SE (IDRI, Seattle, Washington, USA) efficiently treated patients with CL or ML and induced protective immunity in healthy volunteers [68–70]. This vaccine is also safe and tolerated [71]. Among the other adjuvanted artificial proteins, LEISH-F2+MPL-SE and LEISH-F3+GL-SE (glucopyranosyl lipid A-stable oil-in-water nanoemulsion) showed promising results against infection [68–70]. A NS recombinant vaccine, consisting of enzyme nucleoside hydrolase (NH) and sterol 24-c-methyltransferase (SMT) and adjuvanted with “glucopyranosyl lipid A-stable oil-in-water nanoemulsion” (GLA-SE), is also in clinical trial phase [51].
Genetically modified parasite vaccines
In Leishmania vaccine research, live attenuated vaccines made by genetic modifications are another research topic [16]. In this strategy, the parasite genes responsible for its survival and/or virulence are modified or deleted. Unlike live virulent parasites, they do not pose any danger associated with infection. However, they ensure that the induction of immune responses is consistent with protection, since they closely mimic natural infection [72].
Various live attenuated vaccine strains of L. major, L. mexicana, L. amazonensis, and L. donovani have been produced by deleting the targeted genes. They were observed to provide significant protection against CL and VL in susceptible mice. Among them, Biopterin transporter 1 (BT1)-deleted L. donovani parasites and A2–rel gene cluster in L. donovani as well as SIR2, Hsp70-II, and KH1 L. infantum null mutants, protected the mice against virulent strains [73–76]. The p27 gene, encoding an amastigote-specific cytochrome C oxidase component, knockout (gene Ldp27−/−) L. donovani reduced parasitic loads and provided long-lasting protection against the development of CL and VL associated with virulent strains [77,78].
Leishmania centrin gene-1 is necessary for parasite growth and differentiation. The generation of a potential mutant vaccine candidate appears to be an interesting target. Volpedo et al. [28] reported that immunization with LdCen−/− led to a significant influx of MHC-II-expressing macrophages, resulting in higher levels of IFN-γ+-secreting CD4+ Th1 cells and lower levels of IL-10- and IL-4-sectreting CD4+ Th2 cells. This response ensures protection against the virulent parasites.
In general, the most promising strategic alternative against VL can be claimed to be the use of live, non-pathogenic/genetically engineered strains of these species [26].
Third generation (naked DNA) vaccines
Third-generation vaccines utilize the DNA [51]. In this relatively new approach, naked plasmid DNA or DNA encapsulated in a viral vector is injected intradermally or intramuscularly [28,79]. DNA vaccines are safe because they do not contain any pathogenic organisms that may revert virulence [79]. They also efficiently induce interferon-gamma production and dendritic cell activation, which protects against Leishmania infection [80].
DNA vaccines can consist of genes encoding single antigens, such as gp63, LACK, or PSA-2. They can also include multiple genes encoding various antigens such as TSA, KMP11, A-2, NH36, LmSTI1, cysteine proteases, and histones [51]. To increase the immunogenicity of DNA vaccines, these vaccines were primed by boosting the associated protein expressed on a recombinant virus-like modified vaccinia virus Ankara [81]. This strategy selectively elicits a wide range of CD8+ T cells specific for Leishmania antigens [28,79].
Collectively, DNA vaccines are stable, do not require adjuvants, produce antigens over long periods, can easily be produced in large quantities, and are effective. Nonetheless, they bear some safety concerns, such as the integration of DNA into the mammalian genome, which can result in the induction of autoimmune diseases or cancer [28].
DNA vaccines, known as third-generation vaccines, are of particular interest because they can effectively induce both CD8+ and CD4+ T cells, produce long-lived antigens and properly folded polypeptides, etc. [82]. Recently, a phase II study is being conducted to evaluate the therapeutic effects of ChAd63-KH, which consists of 2 genes encoding the L. donovani KMP-11 and HASPB antigens, in patients with persistent post-kala-azar dermal leishmaniasis (PKDL) [83].
New strategy vaccines
Advances in the field of science, in addition to the above-mentioned, have also led to the investigation of new strategies in vaccine development, including certain bioengineering approaches, such as delivery systems and chimeric vaccines, and the use of nonpathogenic Leishmania spp. [19].
Vaccine antigen delivery systems
Drug delivery systems have been extensively studied for the treatment of cancer and infectious diseases [1,88,89]. Nano- particles (NP) are considered ideal vaccine delivery systems. Owing to their structures, vaccine delivery systems present several advantages, such as controlled antigen release, protection of the vaccine antigens from degradation, and site-specific delivery. They can also have adjuvant effect [89]. All these characteristics of NP carriers lead to enhanced bioavailability of antigens, which results in the activation of the immune response. In a study using cationic solid lipid nanoparticles (cSLN) for carrier and delivery, the injection of a fusion gene, pCDNA-A2-CPA-CPB–CTE, enhanced protective cell mediated immunity [90].
Another study demonstrated that vaccination with multifunctionalized PLGA NPs encapsulating sLiAg and/or MPLA provided strong protection against infection with L. infantum [89]. In both studies, the increased production of IFNγ followed by suppression of IL-4 and IL-10 production and very few parasitic loads were considered protective markers. In conclusion, nanoparticle carriers of Leishmania antigen vaccine may be a highly effective strategy against leishmaniasis.
Chimeric vaccines
Lage et al. [91] designed an in silico synthetic recombinant vaccine, named ChimeraT. It contained specific T-cell epitopes from Leishmania prohibitin, eukaryotic initiation factor 5a, and hypothetical LiHyp1 and LiHyp2 proteins. After injecting ChimeraT with saponin as an adjuvant, a Th1-type immune response was induced and BALB/c mice were protected against L. infantum infection [91]. In another study, the F1F3 chimera protein (C-terminal domain of nucleoside hydrolase NH36) showed a strong reduction in ear lesion size induced by L. braziliensis. It also promoted the highest CD4+ and CD8+ cytokine-secreting T cell responses, with predominant frequencies of multifunctional CD4+ and CD8+IL-2+TNF-α+IFN-γ+ T cells [92]. Thus, chimeric proteins could be considered potential vaccine candidates to protect against human diseases.
Nonpathogen Leishmania spp. in vaccination
L. tarentolae, isolated from a reptile animal, is a nonpathogenic Leishmania specie in humans [93,94]. In contrast, this parasite activates the dendritic cell maturation process, which results in the production of interferon gamma and induction of a Th1-type immune response. Furthermore, it mimics the natural development of immunity better than other strategies as an important advantage [89]. Breton et al. [95] also observed that intraperitoneally injected L. tarentolae elicited protective immunity against L. donovani in BALB/C mice. In this regard, they proposed that L. tarentolae is a promising live vaccine candidate against Leishmania infections, without causing any infection in humans.
Breton et al. [95] proposed the idea that it can be improved by generating recombinant L. tarentolae expressing selected Leishmania immunodominant epitopes or by combining the L. tarentolae recombinant parasite with a DNA vaccine as part of a prime-boost strategy to elicit more effective and long-lasting protection. In fact, in a parallel study with a cSLN carrier of the pcDNA-A2-CPA-CPB-CTE fusion gene by Taslime et al. [90], it was reported that recombinant L. tarentolae-A2-CPA-CPB-CTE vaccination was protective against L. infantum infection in BALB/c mice.
Viral-like particle vaccines
One of the latest approaches to developing a safe and cost-effective large-scale vaccine is the use of virus-like particles (VLPs). VLPs are molecules that are morphologically identical to the native virus but cannot replicate because they do not contain viral genetic material (VLPs) [96]. The VLP-based antigen formulation has the potential to generate not only cellular but also humoral immunity that is very similar to that elicited by a natural viral infection VLPs can be produced in different expression systems such as bacterial, yeast, plant, insect or mammalian cells. Panasiuk et al. [86] revealed that VLPs derived from L. tarentolae can induce the production of potent neutralizing antibodies. Maura et al. [97] tested a polyvalent α-Gal, carbohydrates essential for the virulence and viability of many parasites, conjugated to an immunogenic Qβ virus-like particle in a C57BL/6 α-galactosyltransferase knockout mouse model. This vaccine protected knockout mice against L. infantum and L. amazonensis, the aetiological agents of visceral and cutaneous leishmaniasis, respectively.
mRNA vaccines
An effective vaccine both should boost the natural innate and adaptive immune response and induce a memory immune response that provides long-term protection against infection. Recent research reveals that mRNA vaccines significantly enhance these properties [98]. The mRNA platform aslo allows simultaneous expression of multiple proteins, eliciting immunity against different epitopes from different targets [99]. In a mice model, Duthie et al. [100] observed a significant reduction in the parasite burden in the liver by administering F2-RNA as a prime vaccination and then boosting with the recombinant LEISH-F2 protein. RNA vaccine technology has the potential to offer an effective and practical solution to vaccine development. Development of RNA vaccine requires only knowledge of the target gene sequence, eliminating the need for pathogen culture or scale-up recombinant protein production [101].
CONCLUDING REMARK
A safe and efficacious vaccine is urgently required to provide long-lasting protective immunity for the control of parasitic infections. Although cell-mediated immunity is known to play a crucial role in host protection, the immunological protective mechanism of leishmaniasis is complicated. While there is currently no licensed vaccine for human leishmaniasis, extensive efforts are underway to develop a variety of vaccine modalities with promising results worldwide. Some of these modalities are in clinical phase and successful results are obtained in terms of safety and immunogenicity. Meanwhile, it is considered that animal vaccines will play an important role in preventing the transmission of Leishmaniasis to humans. Furthermore, several vaccine candidates are being evaluated at different phases of clinical trials, including those in the first, second, and even third generation.
Notes
The authors declare that they have no known competing financial interests or personal relationships that may have influenced the work reported in this paper.