Expression of cytokines and co-stimulatory molecules in the Toxoplasma gondii-infected dendritic cells of C57BL/6 and BALB/c mice
Article information
Abstract
Toxoplasma gondii is an intracellular protozoan parasite which can infect most warm-blooded animals and humans. Among the different mouse models, C57BL/6 mice are more susceptible to T. gondii infection compared to BALB/c mice, and this increased susceptibility has been attributed to various factors, including T-cell responses. Dendritic cells (DCs) are the most prominent type of antigen-presenting cells and regulate the host immune response, including the response of T-cells. However, differences in the DC responses of these mouse strains to T. gondii infection have yet to be characterized. In this study, we cultured bone marrow-derived DCs (BMDCs) from BALB/c and C57BL/6 mice. These cells were infected with T. gondii. The activation of the BMDCs was assessed based on the expression of cell surface markers and cytokines. In the BMDCs of both mouse strains, we detected significant increases in the expression of cell surface T-cell co-stimulatory molecules (major histocompatibility complex (MHC) II, CD40, CD80, and CD86) and cytokines (tumor necrosis factor (TNF)-α, interferon (IFN)-γ, interleukin (IL)-12p40, IL-1β, and IL-10) from 3 h post-T. gondii infection. The expression of MHC II, CD40, CD80, CD86, IFN-γ, IL-12p40, and IL-1β was significantly higher in the T. gondii-infected BMDCs obtained from the C57BL/6 mice than in those from the BALB/c mice. These findings indicate that differences in the activation status of the BMDCs in the BALB/c and C57BL/6 mice may account for their differential susceptibility to T. gondii.
Introduction
Toxoplasma gondii is an obligate intracellular protozoan parasite which can infect most warm-blooded animals and humans [1]. Although to date, only a single species of this parasite has been identified, there is a marked variation in susceptibility according to its host [2–5]. Among the different mouse models, C57BL/6 mice have been determined to be more susceptible than BALB/c mice to T. gondii infection [2–5]. This difference in susceptibility has been ascribed to multiple factors, including genes associated with the major histocompatibility complex (MHC) [4] and the cytokine storm associated with CD4+ T-cell dependent interferon-γ (IFN-γ) [5]. However, the precise cause of the difference in susceptibility of the different mouse strains to T. gondii infection remains unclear, including the associated cellular immunity control.
Antigen-presenting cells (APCs) play an essential role in the process of cellular immunity, among which dendritic cells (DCs), which can induce innate and adaptive immune responses, have been identified as the most important APC type in the immune system [6]. The maturation of DCs occurs when antigen fragments are presented on the cell surface of immature DCs using MHC molecules, and these cells upregulate co-stimulatory molecules, such as CD40, CD80, and CD86, thereby markedly enhancing their ability to activate T-cells [6]. T. gondii exploits DCs to disseminate throughout the body, produce pro-inflammatory cytokines, and presenting its antigens to the immune system, and in doing so, it is able subvert their signaling pathways and evade detection [7]. DCs play a vital role in the resistance of murine hosts to T. gondii, and mice with depleted DCs have been found to be more susceptible to T. gondii infection [8,9]. The differences in the DC responses are attributable to the genetic susceptibility or resistance of the mouse strains to Trypanosoma cruzi [10], Leishmania major [11], and Coccidioides posadasii [12]. However, no information is currently regarding the activation status or immune response against T. gondii among the DCs derived from susceptible and resistant mouse strains.
Thus, to investigate the activation status of the DCs derived from genetically susceptible or resistant mouse strains in response to T. gondii infection, we cultured bone marrow-derived DCs (BMDCs) from BALB/c and C57BL/6 mice, infected these with T. gondii, and evaluated the expression of the cell surface T-cell co-stimulatory molecules (MHC II, CD40, CD80, and CD86) and cytokines (tumor necrosis factor (TNF)-α, IFN-γ, interleukin (IL)-12p40, IL-1β, IL-10, and transforming growth factor (TGF)-β) based on flow cytometry and quantitative real-time polymerase chain reaction (qRT-PCR) analysis.
Materials and Methods
Survival monitoring of T. gondii-infected mice
C57BL/6 and BALB/c mice (n=5 per group) were obtained from Damool Science, Daejeon, Korea. Each mouse was orally infected with 100 cysts of the T. gondii ME49 strain, and survival was monitored every day for 30 day after infection. All animal-related procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Chungnam National University College of Medicine (Approval no. 202012A-CNU-200). All procedures were conducted in accordance with the relevant guidelines.
Culture and isolation of BMDCs
BMDCs were generated as previously described [9]. Briefly, the tibias and femurs of the mice were disinfected with 70% ethanol for 5 min and washed with phosphate-buffered saline (PBS). Both ends of the bone were cut off with scissors, and the bone marrow cavity was rinsed with sterile PBS using a 1 ml syringe. On day 0, the bone marrow cells were seeded at a density of 4×106 cells per 90×15 mm Petri dish with 10 ml of complete medium (RPMI 1640 medium supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin; WelGENE, Gyeongsan, Gyeongsangbuk-do, Korea), 2-mercaptoethanol (Gibco BRL, Grand Island, NY, USA), 10% fetal bovine serum (FBS), and granulocyte-macrophage colony stimulating factor (GM-CSF; 20 ng/ml; PeproTech, Rocky Hill, NJ, USA) in combination with IL-4 (1 ng/ml; PeproTech). On day 3, 10 ml of fresh complete medium containing GM-CSF and IL-4 was added to the Petri dishes. On day 6, non-nonadherent and loosely adherent cells were collected by gently pipetting the medium against the dish plate. The collected cells were seeded at a density of 2×106 cells per 24-well plate and were used for subsequent experiments.
In vitro T. gondii infection of BMDCs
The BMDCs were suspended in RPMI 1640 medium containing 10% FBS and antibiotics at 37°C in 5% CO2, and infected with freshly harvested live T. gondii tachyzoites at a multiplicity of infection (MOI) of 1. For comparison, BMDCs were also incubated with 100 ng/ml lipopolysaccharide (LPS; Sigma, St. Louis, MO, USA). The BMDCs were treated with live T. gondii tachyzoites for 3, 6, 18, and 24 h, and LPS for 3 and 24 h.
Phenotypic analysis of BMDCs by flow cytometry
The BMDCs were stained with LIVE/DEAD Fixable Dead Cell Staining dye (Life Technologies, Carlsbad, CA, USA) to discriminate between the live and dead cells. The BMDCs were stained with anti-CD40 fluorescein isothiocyanate (FITC), anti-CD86 PE, anti-MHC II PerCP-Cy5.5 (BD Biosciences, San Jose, CA, USA), anti-CD80 PE-Cy7 (eBioscience, Santa Clara, CA, USA), anti-CD11c APC (Tonbo Biosciences, San Diego, CA, USA), and anti-CD11b APC-H7 (BioLegend, San Diego, CA, USA). The samples were run on a BD Canto II flow cytometer (BD Biosciences), and the data were analyzed using FlowJo software (Treestar, Ashland, OR, USA).
Gene expression of cytokines by qRT-PCR
Total RNA was extracted from the BMDCs using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and reverse transcribed to cDNA using the ReverTra Ace RT Kit (Toyobo, Osaka, Japan). PCR was performed in triplicate on a Step One Plus instrument (Applied Biosystems, Waltham, MA, USA). The PCR reagents were obtained from Applied Biosystems, and each sample was run in triplicate in a 20 μl reaction. The PCR conditions were as follows: initial denaturation and enzyme activation at 95°C for 10 min and 40 cycles of 95°C for 15 sec, 60°C for 60 sec, and 72°C for 30 sec. The expression level of the target gene was normalized to that of the housekeeping gene (β-actin) for each sample. The PCR primers used are shown in Supplementary Table S1.
Statistical analysis
The results are expressed as the mean±standard deviation (SD) of at least 3 independent experiments. Significant differences between groups were determined using the two-tailed unpaired Student’s t-test. Statistical significance was set at P<0.05.
Results
Survival of T. gondii-infected BALB/c and C57BL/6 mice
The survival of the C57BL/6 and BALB/c mice infected with T. gondii was monitored over a 30 day post-infection period. All of the C57BL/6 mice had died within 9 day post-infection, whereas all of the BALB/c mice were still alive at the end of the 30 day monitoring period (Fig. 1).
MHC II and co-stimulatory molecule expression was higher in the T. gondii- infected BMDCs from the C57BL/6 mice than from the BALB/c mice
We obtained the BMDCs from uninfected normal C57BL/6 and BALB/c mice. The BMDCs were defined as CD11c+CD11b+ cells (Fig. 2). To compare the activation status of the BMDCs from both mouse strains in response to live T. gondii, we initially investigated the expression of MHC II and co-stimulatory molecules (CD40, CD80, and CD86) via flow cytometry.
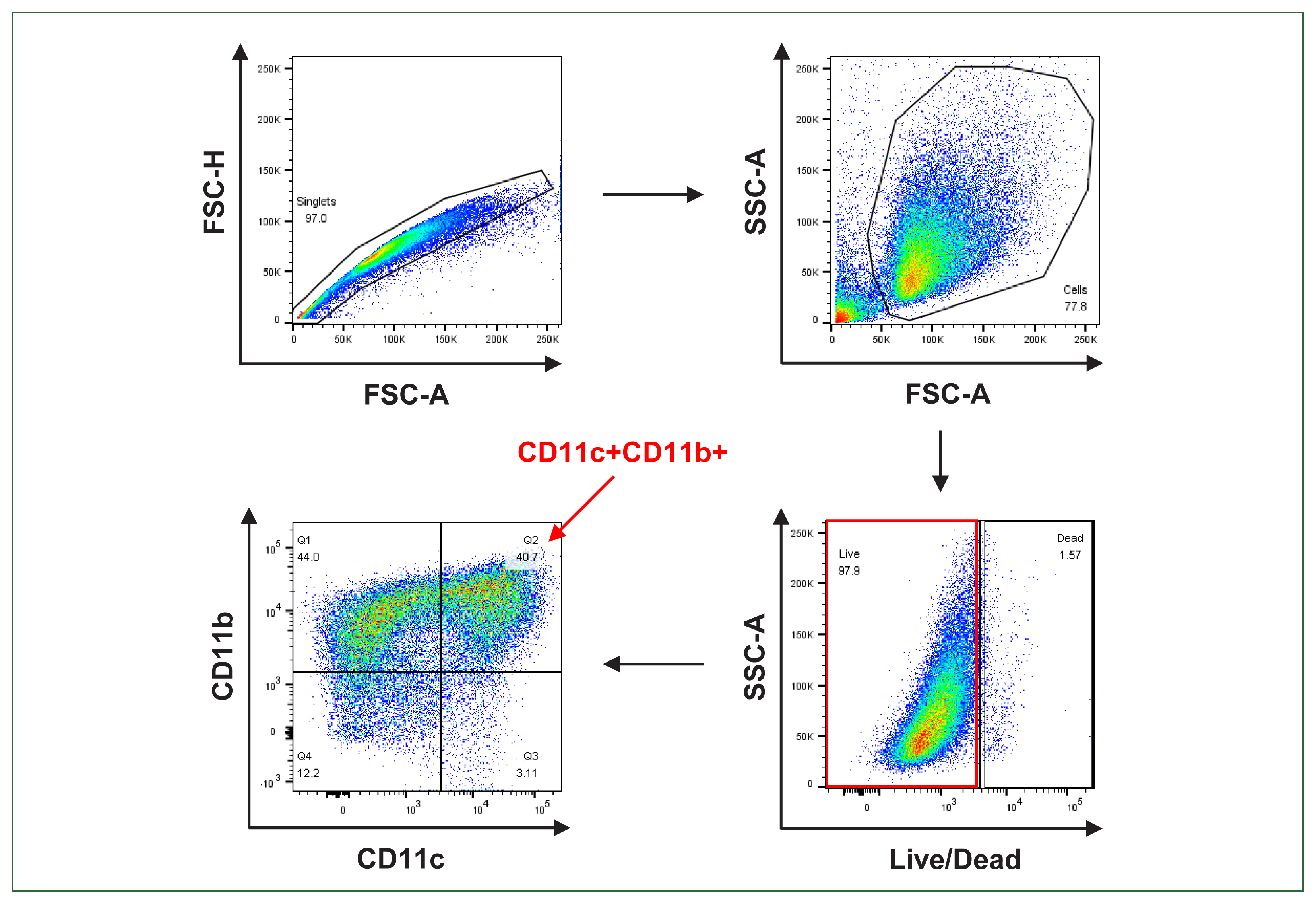
Gating strategy for the bone marrow-derived dendritic cell (BMDCs) analysis. Singlet cells were gated along the diagonal when the forward scatter height (FSC-H) versus forward scatter area (FSC-A) was plotted. Cells were gated when the side scatter area (SSC-A) versus the FSC-A was plotted. Live cells were live/dead Amcyan negative when the SSC-A was plotted versus the live/dead Amcyan. The CD11c+CD11b+ cells were discriminated by plotting CD11b versus CD11c. The BMDCs were defined as CD11c+CD11b+.
Although the expression of MHC II in the BMDCs derived from the BALB/c mice did not significantly differ following T. gondii infection, we detected an increase in MHC II expression in the C57BL/6 BMDCs in response to infection (P=0.067). Moreover, during the entire experimental period, the MHC II expression in T. gondii-infected BMDCs from the C57BL/6 mice was significantly higher than that in the BMDCs from the BALB/c mice (Fig. 3A).
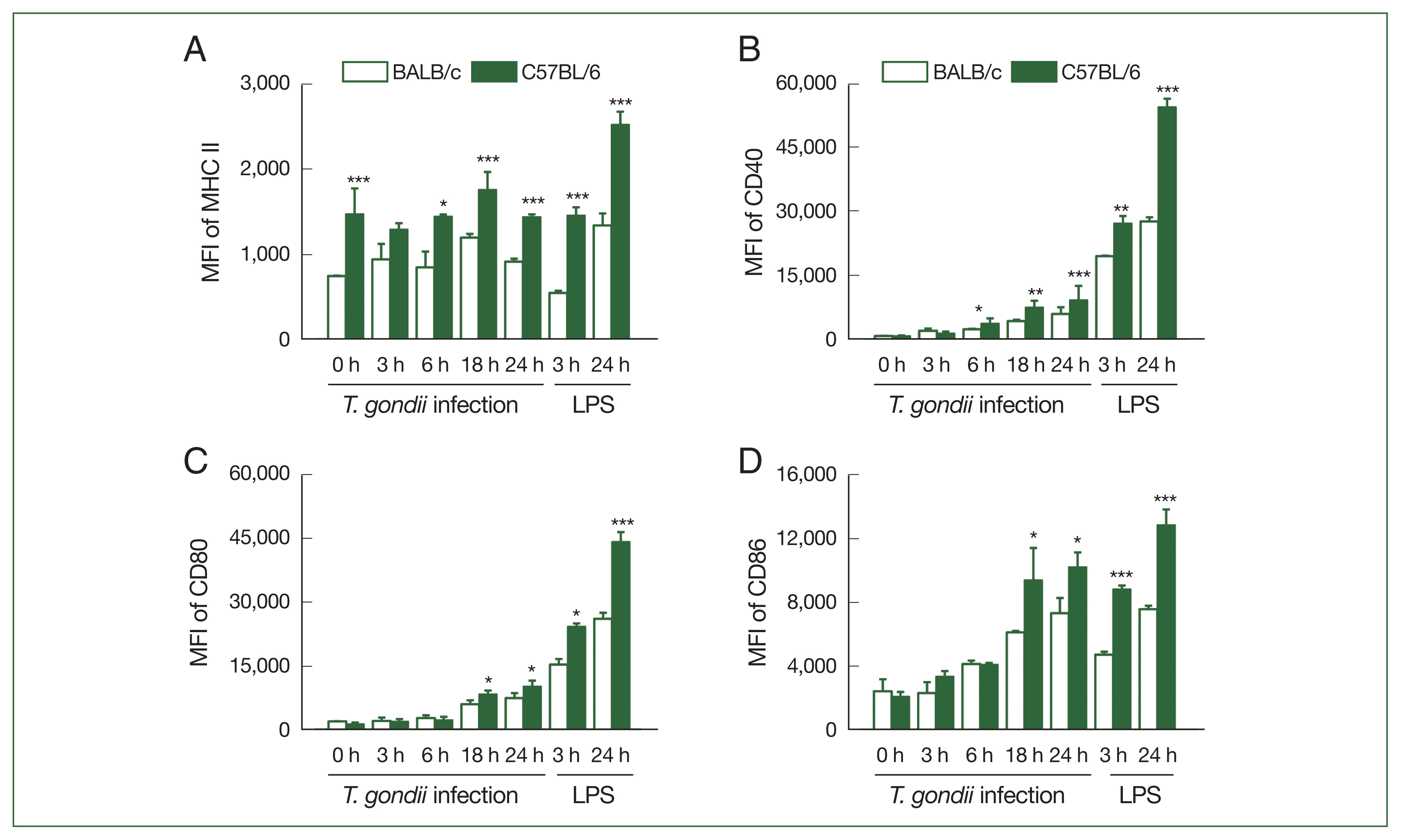
Expression levels of MHC II and co-stimulatory molecules in T. gondii-infected bone marrow-derived dendritic cell (BMDCs) were higher in the C57BL/6 than in the BALB/c mice. The BMDCs were generated from murine bone marrow cultured for 6 days with granulocyte-macrophage colony stimulating factor (GM-CSF)- and interleukin (IL)-4-enriched medium and were then treated with T. gondii at an multiplicity of infection (MOI) of 1 for 3, 6, 18, and 24 h or 100 ng/ml lipopolysaccharide (LPS) for 3 and 24 h. The expression levels of MHC II (A), CD40 (B), CD80 (C), and CD86 (D) were analyzed via flow cytometry and represented the mean fluorescence intensity (MFI) of each group (n=5 mice per group). Data are shown as the mean±SD. *P<0.05, **P<0.01, and ***P<0.001 as compared to the BALB/c mice under the same conditions.
In the BMDCs of both mouse strains, we detected a gradual increase in the expression of CD40, CD80, and CD86 following T. gondii infection, which peaked at 24 h post-infection. The expression of CD40 in the T. gondii-infected BMDCs from the C57BL/6 mice was found to be significantly higher than that in the T. gondii-infected BMDCs from the BALB/c mice from 6 h post-infection (Fig. 3B) (P<0.01). In contrast, the CD80 and CD86 expression in the T. gondii-infected BMDCs from the C57BL/6 mice was observed to be significantly higher than those in the infected BMDCs from the BALB/c mice from 18 h post-infection (Fig. 3C, D).
LPS promotes an increase in the expression of co-stimulatory proteins and induces the production of polarizing Th1 and Th2 cytokines/chemokines in DCs [13], thus we used LPS as a positive stimulant of the BMDCs from both mouse strains. The expression of MHC II, CD40, CD80, and CD86 in the T. gondii-infected BMDCs from the C57BL/6 mice was found to be significantly higher than that in the T. gondii-infected BMDCs from the BALB/c mice at 3 and 24 h post-infection. In the LPS-treated cells, we detected a significantly higher expression of CD40 and CD80 than in the T. gondii-infected cells, although this was not observed for MHC II and CD86 (Fig. 3A–D).
Cytokine expression was higher in the T. gondii-infected BMDCs from the C57BL/6 mice than in the BALB/c mice
We subsequently investigated the cytokine production in the T. gondii-infected BMDCs derived from the C57BL/6 and BALB/c mice based on qRT-PCR analysis (Fig. 4). In both the mouse strains, compared to the uninfected BMDCs, we detected an significant increase in the mRNA expression of TNF-α, IFN-γ, IL-12p40, IL-1β, and IL-10 in the BMDCs from 3 h post-infection, with the mRNA expression of IFN-γ, IL-12p40, IL-1β, and TGF-β in the C57BL/6 mice being significantly higher than that of the BALB/c mice (Fig. 4A-F). In both strains, the mRNA expression of TNF-α peaked at 3 h post-infection and thereafter declined (Fig. 4A). In contrast, we detected a reduction in the TGF-β mRNA expression level in the BALB/c BMDCs in response to T. gondii infection, whereas a slight increase was observed in C57BL/6 BMDCs (Fig. 4F).
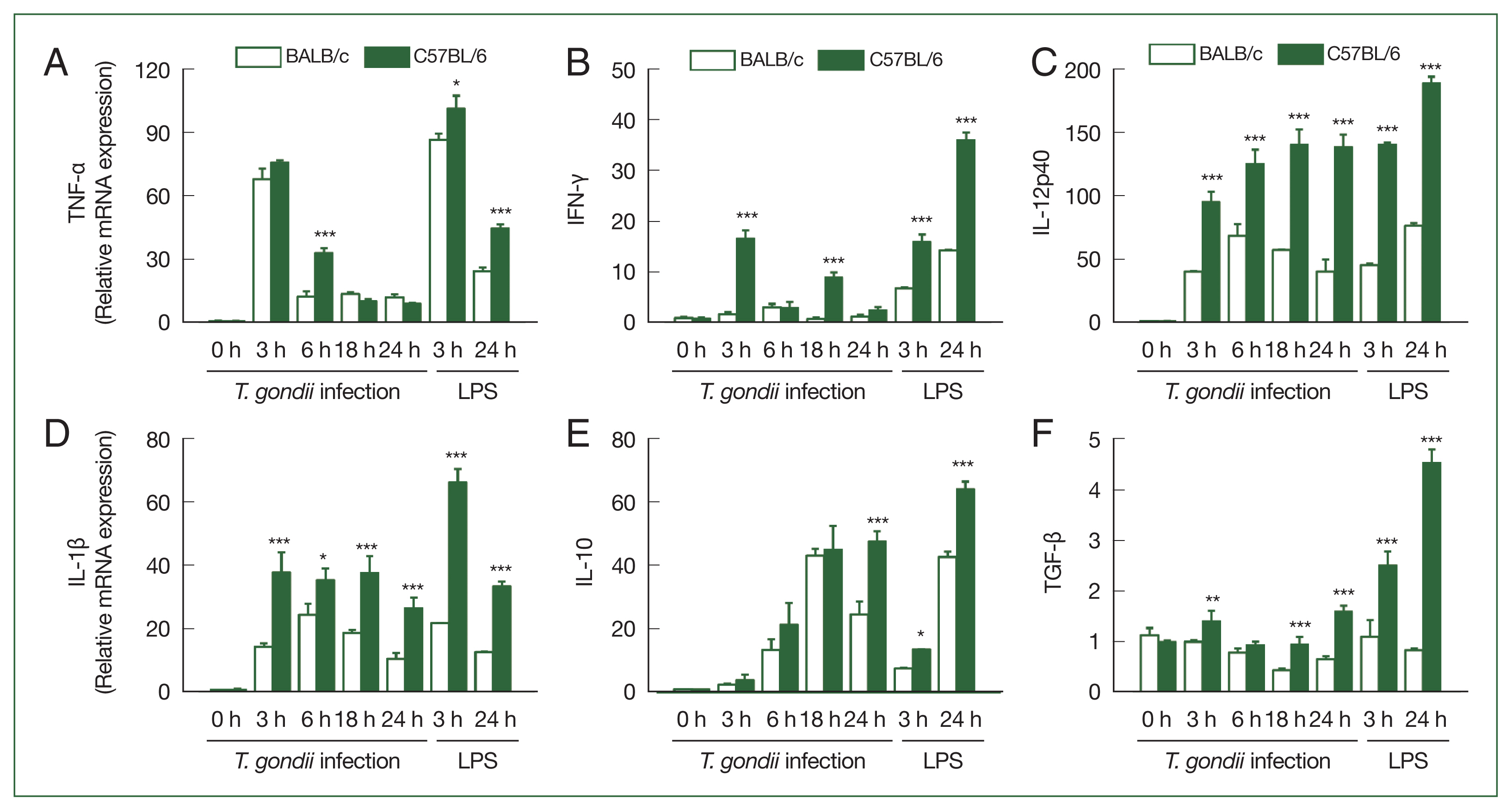
Cytokine expression levels in T. gondii-infected BMDCs were higher in the C57BL/6 than in the BALB/c mice. The BMDCs were treated with T. gondii at a MOI of 1 for 3, 6, 18, and 24 h or 100 ng/ml LPS for 3 and 24 h. The mRNA expression levels of tumor necrosis factor (TNF)-α (A), interferon (IFN)-γ (B), interleukin (IL)-12p40 (C), IL-1β (D), IL-10 (E), and transforming growth factor (TGF)-β (F) in the BMDCs were measured via quantitative real-time polymerase chain reaction (qRT-PCR). Y axis: relative mRNA expression levels compared to the uninfected control under the same conditions, X axis: hours post-infection. Data are shown as the mean±SD of each group (n=5 mice per group). *P<0.05, **P<0.01, and ***P<0.001 as compared to the BALB/c mice under the same conditions.
In the LPS-treated BMDCs, significantly higher mRNA expression levels of TNF-α, IFN-γ, IL-12p40, IL-1β, IL-10, and TGF-β were found in the C57BL/6 mice compared to the BALB/c mice. In the case of TNF-α, IFN-γ, and TGF-β the mRNA expression levels were significantly higher in the LPS-treated cells than in the T. gondii-infected cells, although this was not observed for the mRNA expression levels IL-12p40, IL-1β, or IL-10 (Fig. 4A–F).
Discussion
In this study, we investigated the activation status of the BMDCs derived from the BALB/c and C57BL/6 mice in response to T. gondii infection. Our findings revealed that the different responses of the BMDCs to T. gondii infection in these 2 inbred mouse strains which differ with respect to their susceptibility to T. gondii infection. We found that the survival of the C57BL/6 mice was considerably shorter than that of the BALB/c mice following infection with T. gondii Me49 cysts. This is consistent with previous reports indicating that C57BL/6 mice are more susceptible to T. gondii infection than BALB/c mice (Fig. 1) [2–5]. Having established this different response between the 2 strains, we subsequently evaluated the expression of the cell surface T-cell co-stimulatory molecules and cytokines in the BMDCs derived from the 2 mouse strains.
DCs are specialized APCs characterized by their unique capacity to initiate and modulate immune responses via their priming of naïve T-cells, the activation of which is dependent on antigens, co-stimulatory molecules, and inflammatory cytokines [6]. Upon stimulation, DCs undergo several morphological, phenotypic, and functional changes during their maturation process [6]. In this regard, a previous transcriptomic analysis of T. gondii-infected BMDCs revealed significant alterations in the transcripts associated with cellular metabolism, activation of T-cells, and inflammation-mediated chemokine and cytokine signaling pathways [14]. In addition, T. gondii-induced activation of human DCs is associated with the T-cell production of IFN-γ mediated via the CD40-CD40L-dependent release of IL-12 and through the CD80/CD86-CD28 interaction in the absence of bioactive IL-12 [15]. The findings of these studies indicate that in response to T. gondii infection, DCs may play a key role in the association between the innate immune response and adaptive immunity.
In this study, we analyzed the response of the BMDC cell surface markers, including MHC II and the co-stimulatory molecules CD40, CD80, and CD86 to T. gondii infection. We detected significant increases in the expression of these markers in the BMDCs of the C57BL/6 and BALB/c mice following T. gondii infection (Fig. 3). Interestingly, the expression of the cell surface markers was found to be significantly higher in the T. gondii-infected BMDCs of the C57BL/6 mice than in the BALB/c mice. These observations indicate that whereas T. gondii induces the activation of BMDCs in both mouse strains, but compared with the BALB/c BMDCs, those in the C57BL/6 mice were more effective in capturing and processing the antigens to elicit specific T-cell effector responses following T. gondii infection.
Another hallmark of DC activation is a shift in the profile of the secreted cytokines. Mature DCs secrete inflammatory cytokines (IL-12, IL-1β, and TNF-α) and anti-inflammatory cytokines (IL-10 and TGF-β) and present antigens to naïve T-cells for activation [6,16]. In this regard, as previously reported, the production of pro-inflammatory cytokines, such as IL-1α, IL-1β, IL-6, IL-10, and TNF-α, was significantly increased in the BALB/c mouse splenic CD11c+ DCs at week 1 post-infection [17]. It has been shown that in response to stimulation with Toxoplasma antigens or live parasites, the T-cells derived from the C57BL/6 mice preferentially produced Th1 cytokines with high IFN-γ and low IL-4 levels, whereas those from the BALB/c mice typically produced Th2 cytokines with low IFN-γ and high IL-4 levels [3,18]. Similar T-cell responses have been observed when these mouse strains were infected with Leishmania major [19]. Consistent with the findings of Watanabe et al. [3], we observed that the IFN-γ, IL-12p40, IL-1β, and TGF-β mRNA expression levels were significantly higher in the BMDCs obtained from the C57BL/6 mice than in the BALB/c mice from 3 h post-infection (Fig. 4). Our findings indicate that T. gondii infection induces cytokine profiles polarized to Th1 and Th2 immune responses in the BMDCs of the C57BL/6 and BALB/c mice, respectively.
In conclusion, our findings in this study revealed that the differences in the activation status of the BMDCs in the BALB/c and C57BL/6 mice could be a factor contributing to the differences in the susceptibility of these 2 mouse strains to T. gondii infection.
Supplementary Information
Acknowledgment
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (2022R1A2B5B01001641, 2022K2A9A2A06037100).
Notes
The authors declare no conflict of interest related to this study.
Author contributions
Conceptualization: Lee JH, Lee YH
Data curation: Lee JH, Lee YH
Formal analysis: Lee JH, Lee YH
Funding acquisition: Lee YH
Investigation: Lee JH, Yuk JM, Cha GH, Lee YH
Methodology: Lee JH, Lee YH
Project administration: Lee YH
Resources: Lee YH
Software: Lee JH
Supervision: Yuk JM, Cha GH
Validation: Lee JH, Yuk JM, Cha GH, Lee YH
Visualization: Lee JH
Writing – original draft: Lee YH

