Trematode metacercariae and adults in cyprinoid fish from Khun Thale Swamp in Surat Thani province, Thailand
Article information
Abstract
The present study aimed to determine the infection status of trematode metacercariae and adults in cyprinoid fish from the Khun Thale Swamp in Surat Thani, Southern Thailand, with epidemiologic and faunistic viewpoints. In 2020, 577 fish in 15 species were collected in the summer (February–April) and rainy (September–November) seasons. Fish were individually examined for trematode metacercariae in the whole body and adults in the gastrointestinal tract using a stereomicroscope. Three species of digenetic trematode metacercariae, i.e., Haplorchis taichui, Haplorchoides mehrai, and Centrocestus formosanus, were detected in the muscle, fin, and/or scale of fish. Two species of adult flukes, including Rohdella siamensis and Helostomatis cyprinorum, were collected in the intestines. The prevalence of overall trematode infections was 32.4% (187/577 fish), which was higher in the rainy season (41.4%; 118/285) than in the summer season (23.6%; 69/292). The metacercariae of H. taichui and H. mehrai were detected in 7 fish species each, and those of C. formosanus were found only in Rasbora toneri. The aspidogastrean trematode R. siamensis (adult) was detected in Babonymus gonionotus. A digenean species, H. cyprinorum (adult), was found in Labiobarbus siamensis and Osteochilus vittatus. The present study has first confirmed that the metacercariae of heterophyid flukes, including H. taichui, H. mehrai, and C. formosanus, and adults of R. siamensis (Aspidogastrea) and H. cyprinorum (Digenea) are infected in some species of the cyprinoid fish from the Khun Thale Swamp in Surat Thani, Thailand.
Introduction
Trematodes in fish are subclassified into Digenea and Aspidogastrea [1,2]. Digenean trematodes can cause public health and veterinary problems. Among them, heterophyids including Haplorchis spp., Haplorchoides spp., and Centrocestus spp., take freshwater or brackish water fish as their second intermediate host. Birds and mammals, including humans, are the definitive hosts for Haplorchis spp. and Centrocestus spp., whereas fish-eating fish for Haplorchoides spp. [1,2].
Freshwater or marine fish or other aquatic animals, including mussels, are infected by adult flukes of Aspidogastrea (Platyhelminthes: Trematoda) [1–5]. Among them, the genus Rohdella was first recorded in Thailand in 1984 with Rohdella siamensis as a new (type) species found in the cyprinoid fish Hypsibarbus wetmorei (syn. Barbus daruphani, Puntius daruphani) and Osteochilus melanopleura [1–5]. It was never found again in Thailand or elsewhere [6], and thus research should be performed to extend its geographical distribution and host diversity. In 1934, the genus Helostomatis (Digenea: Paramphistomatidae) was reported with H. helostomatis as the type in Indonesia [7]. In 1984, H. cyprinorum was recorded as a new species in Malaysia from the intestine of cyprinoid fish (Labiobarbus festiva and Osteochilus melanopleura) [2,7,8]. Helostomatis sp. was reported in Labiobarbus siamensis fish from the northeastern part of Thailand [9]. However, its species’ name was not determined, and it was never found again in Thailand [6]. Thus, research is needed from a faunistic perspective to investigate its geographical distribution and host diversity.
Various trematode species have been reported in freshwater fish in Thailand, particularly cyprinoid fish [9–17], and studies should be continued to extend our knowledge of fish trematodes in addition to their public health and faunistic significance. The Khun Thale Swamp is located in the lower part of the Tapi River in Mueang, Surat Thani, Thailand. It flows through a mangrove forest into an estuary at Bandon Bay, which is a part of the Gulf of Thailand. This swamp is an important resource for agriculture, fisheries, tourism, and biodiversity, including fish [18]. However, surveys on the infection status of trematode metacercariae and adults in cyprinoid fish were never conducted in this swamp until now. Therefore, the present study aimed to determine the infection status of trematode metacercariae and adults in cyprinoid fish from this swamp.
Materials and Methods
Study site
The study was conducted in the Khun Thale Swamp, Surat Thani, Southern Thailand (9°4′ 3″N 99°19′52″E). The swamp is a part of the Tapi River’s catchment area (Supplementary Fig. S1).
Fish collection
Freshwater fish belonging to the family Cyprinidae (15 species) were collected from a local fisherman at the Khun Thale Swamp, Surat Thani, Thailand. In 2020, 577 fish were collected for 6 months during the summer (February–April) and rainy (September–November) seasons. The species of each fish were identified and then examined for trematode metacercariae and adults.
Examination of trematode metacercariae and adults
The scales and fins of the fish were removed, placed in a Petri dish containing 0.85% saline solution, and examined under a stereomicroscope. The gill filaments were dissected and placed in 0.85% saline for the parasites infecting gills. The fish’s body was examined after dissecting the internal organs. Briefly, scissors were used to cut open the fish, from the anus to the posterior part of the operculum. Internal organs, such as the liver, gall bladder, stomach, and intestine, were dissected and placed in separate Petri dishes containing 0.85% saline and then examined using a stereomicroscope. Caudal fin muscle tissues were minced to detect metacercariae, as reported previously [14,19]. The metacercarial stage was isolated using 1% acid pepsin solution for 2 h at 36°C, and the digested material was passed through a sieve tube and rinsed with 0.85% saline [14,16,20]. The intestines of the fish were examined for adult flukes. The collected parasite specimens were rinsed in 0.85% saline, fixed in 4% formalin, and set between a slide and coverslip with light pressure. The adult specimens were stained by acetocarmine or hematoxylin, dehydrated in an alcohol series, cleared in xylene, and mounted in Permount [14]. The parasite species were morphologically identified under a compound microscope following the related literature [1,2,7,14,20,21]. The prevalence and the mean intensity of parasite species were calculated following the method of Margolis et al. [22]. An Olympus CX31 microscope (Olympus, Tokyo, Japan) with a drawing tube (UDA60) was used to make morphometric data and illustrations. Measurements are given in millimeters as the means with ranges in parentheses.
Results
Prevalence of trematodes in fish
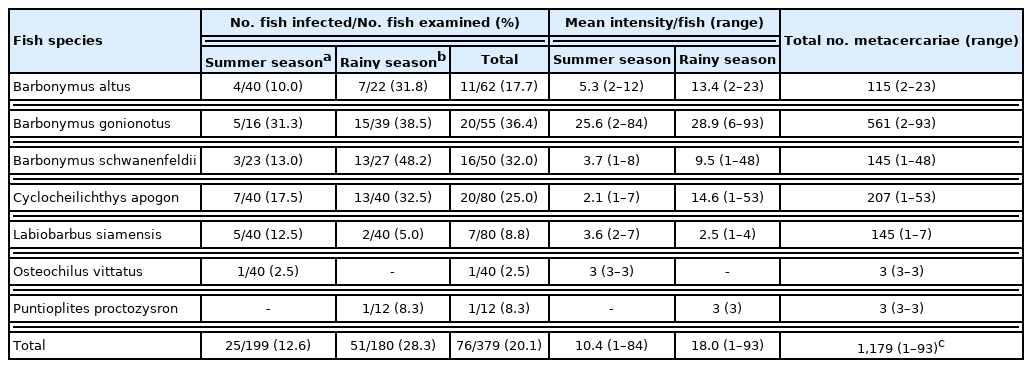
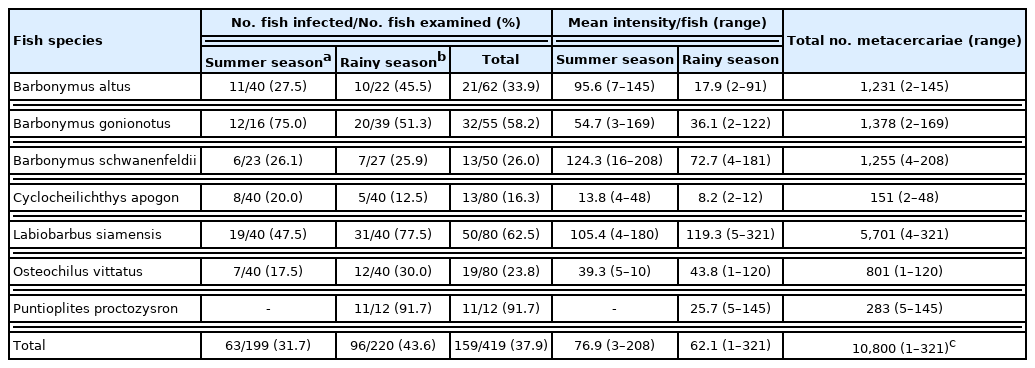
Out of 15 species of cyprinoid fish examined, 8 were found infected with larval or adult trematodes. The detected metacercariae included 3 species belonging to the Heterophyidae family, i.e., Haplorchis taichui, Haplorchoides mehrai, and Centrocestus formosanus (syn. Centrocestus caninus). The adult trematodes included 2 species, Aspidogastrea (Rohdella siamensis) and Digenea (Helostomatis cyprinorum) (Fig. 1A–F, Fig. 2A–D).

Encysted (A) and excysted (B) metacercariae of Haplorchis taichui collected from the muscle of Barbonymus gonionotus. Encysted (C) and excysted (D) metacercariae of Haplorchoides mehrai collected from the scale of Labiobarbus siamensis. Encysted (E) and excysted (F) metacercariae of Centrocestus formosanus collected from the gill of Rasbora toneri. Scale bars=0.1 mm.
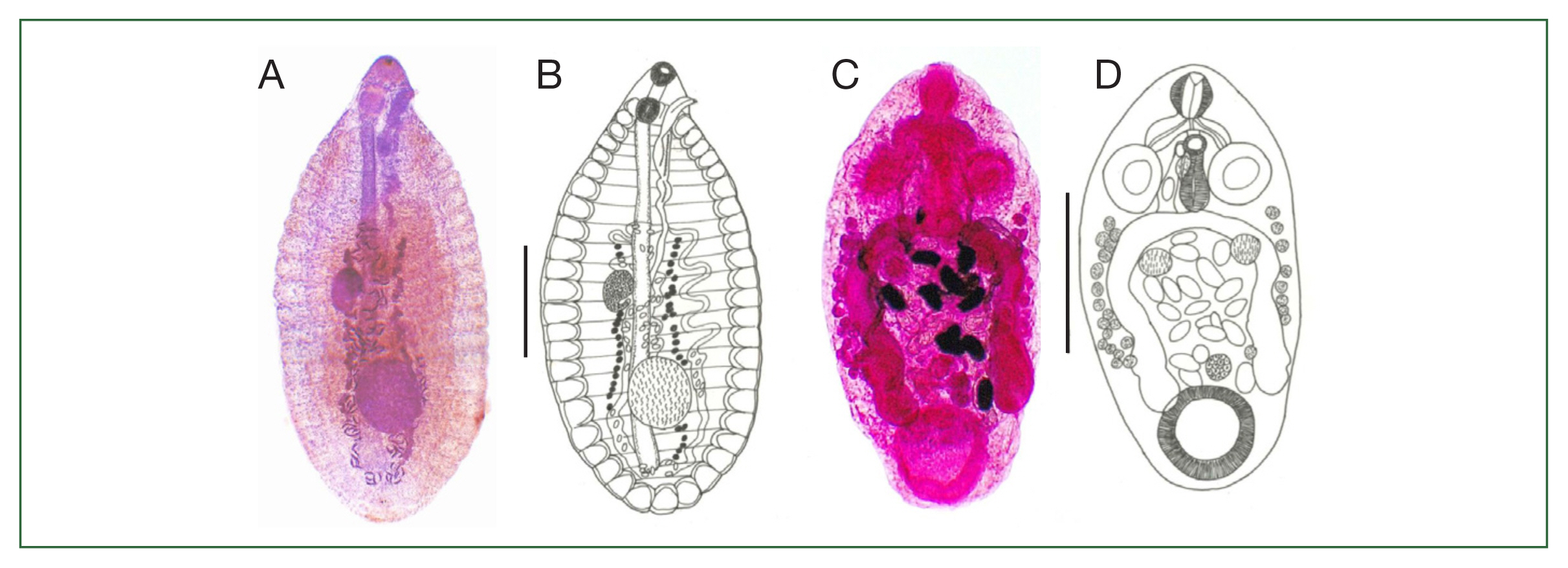
Adult Rohdella siamensis (A, B) collected from the intestine of Barbonymus gonionotus (A, acetocarmine-stained specimen; B, line drawing), and adult Helostomatis cyprinorum (C, D) collected from the intestine of Osteochilus vittatus (C, acetocarmine-stained specimen; D, line drawing). Scale bars=0.1 mm.
The overall prevalence of larval or adult trematodes was 32.4% (187/577), generally (regardless of trematode species) higher in the rainy season (av. 41.4%; 5.0–91.7% by fish species) than in the summer season (average: 23.6%; 15.0–75.0% by fish species) (detailed data not shown). The metacercariae of H. taichui and H. mehrai were found in the muscle and fin and the muscle, fin, and scale of the fish, respectively. The metacercariae of C. formosanus was found in the gills of the fish. The species of adult trematodes (R. siamensis and H. cyprinorum) were found in the intestine of the fish.
H. taichui metacercariae were detected in 7 species of fish (Table 1), with its highest prevalence in B. gonionotus of 36.4% (summer: 31.3% and rainy: 38.5%) with a mean intensity of 25.6 and 28.9 metacercariae/fish in the summer and rainy seasons, respectively. The overall prevalence was 20.1% (summer: 12.6% and rainy: 28.3%), with 1,179 detected metacercariae in 76 infected fish (average: 15.5 metacercariae per fish; 1–93 by individual fish) (Table 1). H. mehrai metacercariae were found in 7 species of fish (Table 2), with its highest prevalence in L. siamensis at 62.5% (summer: 47.5% and rainy: 77.5%), with a mean intensity of 105.4 and 119.3 metacercariae/fish in summer and rainy seasons, respectively. The overall prevalence was 37.9% (summer: 31.7% and rainy: 43.6%), with 10,800 detected metacercariae in 159 fish (average: 67.9 metacercariae/fish; 1–321 by individual fish) (Table 2). C. formosanus metacercariae were found only in R. toneri fish with a prevalence of 11.7% (summer: 15.0% and rainy: 5.0%), and mean intensity of 3.2 and 5.0 metacercariae/fish in the summer and rainy seasons, respectively (Table 3).
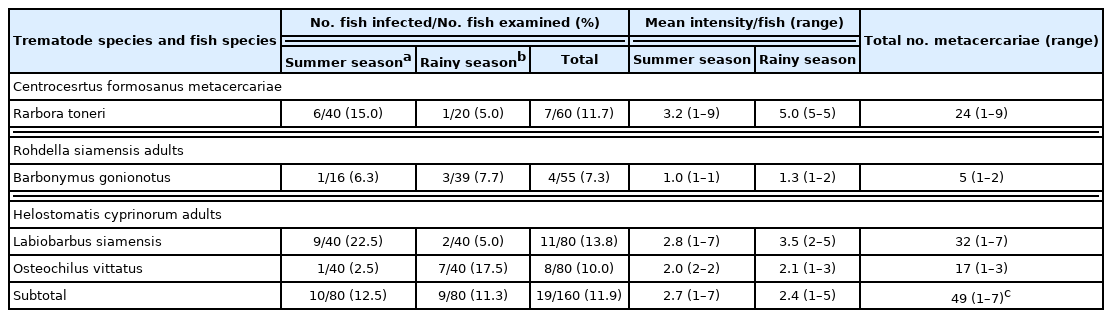
Infection status of Centrocestus formosanus metacercarae and Rohdella siamensis and Helostomatis cyprinorum adults by fish species
The adult flukes of R. siamensis were found only in B. gonionotus fish with a prevalence of 7.3% (6.3% in summer and 7.7% in rainy seasons), and a mean intensity of 1.0 and 1.3 parasites/fish in the summer and rainy seasons, respectively (Table 3). The adults of H. cyprinorum were found in 2 fish species: L. siamensis and O. vittatus, with an overall prevalence of 11.9% (summer: 12.5% and rainy: 11.3% in seasons) and a mean intensity of 2.7 and 2.4 parasites/fish in the summer and rainy seasons, respectively (Table 3).
Measurements and diagnostic criteria for larval and adult trematodes
Haplorchis taichui (metacercaria; Fig. 1A, B): The encysted metacercaria is oval-shaped and is 0.18 (0.18–0.22) mm in length and 0.13 (0.12–0.18) mm in width (n=10). The excysted metacercaria is elongated and oval and is 3.52 (0.32–0.55) mm in length and 1.50 (0.08–0.20) mm in width (n=10). The ventrogenital sac is located ventrally in the middle region of the body and armed with 16 long, chitinous, and crescentic spines, which is a specific characteristic. A single testis is observed.
Haplorchoides mehrai (metacercaria; Fig. 1C, D): The encysted metacercaria is oval-shaped and is 0.17 (0.16–0.20) mm in length, and 0.12 (0.12–0.16) mm in width (n=10). The excysted metacercaria is elongated and is 0.40 (0.35–0.50) mm in length and 0.13 (0.12–0.18) mm in width (n=10). The number of acetabular spines is 19–27 in total, including 5–7 in the anterior, 7–10 in the median, and 7–10 in the posterior groups, which is a specific characteristic of this species. The testis is single.
Centrocestus formosanus (metacercaria; Fig. 1E, F): The encysted metacercaria is oval-shaped and is 0.21 (0.18–0.22) mm in length, and 0.14 (0.12–0.16) mm in width (n=10). The excysted metacercariae are pyriform in shape and measured 0.45 (0.40–0.48) mm in length and 0.14 (0.13–0.15) mm in width (n=10). The oral sucker is surrounded by 32 circumoral spines in 2 rows. The excretory bladder is characteristically X-shaped, situated in the posterior third of the body between the 2 testes.
Rohdella siamensis (adult; Fig. 2A, B): The body is elongate-oval and measured 3.75 (3.10–4.13) mm in length, and 1.63 (1.35–1.88) mm in width (n=4). The holdfast organ at the ventral surface is composed of 23 (22–23) rows of transverse alveoli divided by 3 longitudinal septa, arranged in a marginal ring of 46–48 and 22–23 medial alveoli, which is one of the specific characteristics of this species. The genital pore is sinistro-lateral near the forebody and overlaps the holdfast organ. A single testis is post-ovarian and globular to oval. Eggs are ovoid and measured 0.09 (0.08–0.10) mm in length and 0.04 (0.03–0.05) mm in width.
Helostomatis cyprinorum (adult; Fig. 2C, D): The body is oval, with its posterior part slightly broader than the anterior one and measured 2.80 (1.35–3.90) mm in length and 0.91 (0.68–1.28) mm in width (n=10). Two cecae extend to the posterior 1/5 of the body, having a 3-shape configuration, which is one of its specific characteristics. Two oval-lobulate testes are located pre-ovarian, and the ovary is ovoid, situated between the testes and the ventral sucker. Eggs are ovoid, embryonated, and measured 0.11 (0.08–0.15) mm in length and 0.70 (0.05–0.10) mm in width.
Discussion
The present investigation revealed 4 species of digenetic trematodes (3 in the metacercarial stage and 1 in the adult stage) and 1 species of aspidogastrean trematode (adult stage). Previous workers in Thailand reported 3 species of metacercariae, including C. formosanus, H. taichui, and H. mehrai, in cyprinoid fish [14,23–30]. The metacercariae of H. taichui and C. formosanus can develop into adults in birds and mammals, including humans, and thus they are of zoonotic significance [21,23–32]. These flukes can cause mild to severe gastrointestinal (abdominal pain, diarrhea, weight loss, and lethargy) or irritable bowel syndrome-like symptoms [21,31]. The discovery of H. taichui and C. formosanus metacercariae in cyprinoid fish indicated their potential role as an intermediate host and the source of human infections [19,24,27,31]. Preventive measures for these trematode infections should include proper cooking of cyprinoid fish before consumption.
This study revealed the metacercariae of H. mehrai in 7 species of cyprinoid fish. A previous study reported that these metacercariae encysted in small-sized freshwater fish, including B. gonionotus, B. schwanenfeldii, Cyclocheilichthys repasson, and L. siamensis, in Chiang Mai, Thailand [14]. They can develop into adult flukes in the intestine of large, fish-eating fish, including catfishes Mystus vittatus in India and Hemibagrus nemurus, Hemibagrus wyckioides, and Mystus multiradiatus in Thailand (Khon Kaen, Chiang Mai, and Chiang Rai province) [30]. However, this study only revealed the metacercarial stage.
R. siamensis was reported as a new genus and a new species in 1984 from the intestine of 2 cyprinoid fish species in Thailand [1]. Since then, the literature has described 3 additional species, i.e., R. clariasa, R. anodontiase, and R. amazonica [3–5]. In 1989, R. clariasa was found in the intestine of a Nile fish (Clarias lazera) in Egypt, and in 1993, R. anodontiase was found in the pericardial and nephridial cavities of the mussel (Anodonta rubens) in Nile River, Egypt [5]. R. amazonica was discovered in the intestine of the Amazonian banded puffer (Colomesus psittacus) in Brazil in 2015 [4]. R. siamensis differs from R. clariasa and R. anodontiase in having an eversible hermaphroditic duct and from R. amazonica in having smaller ovary and testes and a longer and more tubular hermaphroditic duct [4]. To our best knowledge, this is the second report on R. siamensis adults in the literature and the first one in Southern Thailand.
Three additional species were recorded after creating the genus Helostomatis (Digenea: Paramphistomatidae) with the type H. helostomatis [7]. H. sakrei was recorded from the fish Labeo calbasu in India in 1937, and H. cirrhini was from the fish Cirrhina mrigala in India in 1970 [7]. H. cyprinorum was recorded as a new species from the intestine of Malaysian cyprinoid fish [7]. H. cyprinorum differs from H. helostomatis and H. sakrei in terms of larger diverticular pouches and 3-shaped caeca [7]. In addition, H. cirrhini and H. sakrei have a trilobed ovary, whereas H. cyprinorum has an unlobed ovary [7]. The present study revealed H. cyprinorum in 2 fish species, including L. siamensis and O. vittatus, which are new host records. This is the first record of H. cyprinorum adults in Thailand (only a record of Helostomatis sp. [9] is available) and the second recorded in the literature.
Generally, the infection patterns of fish trematodes are greatly influenced by seasons, fish species, and the water body type [33,34]. This study revealed a generally higher prevalence of trematodes in the rainy season than in the summer season. A previous study suggested that a lower temperature in the rainy season might induce parasite growth in fish, while a higher temperature in the summer season might reduce their growth [35]. Additionally, fecal contamination would probably be the greatest during the rainy season with a rapid increase in the snail population [10,36].
In conclusion, 5 species of larval or adult trematodes were detected in 8 of 15 examined species of cyprinoid fish in Southern Thailand. Larval trematodes (metacercarial stage) included 2 species (H. taichui and C. formosanus) that can infect birds and mammals, including humans, and thus they are of zoonotic significance, and 1 species (H. mehrai) that can infect large fish-eating fish to become adults. Two adult trematode species (R. siamensis and H. cyprinorum) are the second report in the available literature and new trematode fauna in Southern Thailand.
Supplementary Information
Acknowledgment
This research was carried out with financial support provided by the Prince of Songkla University (grant no. SIT6302002S).
Notes
The authors declare no conflict of interest related to this study.
Author contributions
Conceptualization: Kamchoo K
Data curation: Kamchoo K
Formal analysis: Kamchoo K
Funding acquisition: Kamchoo K
Investigation: Kamchoo K
Methodology: Kamchoo K
Project administration: Kamchoo K
Resources: Kamchoo K
Software: Kamchoo K, Chai JY
Supervision: Chai JY
Validation: Kamchoo K, Chai JY
Visualization: Kamchoo K, Chai JY
Writing – original draft: Kamchoo K
Writing – review & editing: Chai JY