Disease vector occurrence and ecological characteristics of chiggers on the chestnut white-bellied rat Niviventer fulvescens in Southwest China between 2001 and 2019
Article information
Abstract
Chigger mites are the vector of scrub typhus. This study estimates the infestation status and ecological characteristics of chiggers on the chestnut white-bellied rat Niviventer fulvescens in Southwest China between 2001 and 2019. Chiggers were identified under the microscope, and infestation indices were calculated. The Preston’s log-normal model was used to fit the curve of species abundance distribution. A total of 6,557 chiggers were collected in 136 of 342 N. fulvescens rats, showing high overall infestation indices (prevalence=39.8%, mean abundance=19.2, mean intensity=48.2) and high species diversity (S=100, H′=3.0). Leptotrombidium cangjiangense, Neotrombicula japonica, and Ascoschoengastia sifanga were the three dominant chigger species (constituent ratio=42.9%; 2,736/6,384) and exhibited an aggregated distribution among different rat individuals. We identified 100 chigger species, with 3 of them (Leptotrombidium scutellare, Leptotrombidium wenense, and Leptotrombidium deliense) as the main vectors of scrub typhus in China and nine species as potential vectors of this disease. Disease vector occurrence on N. fulvescens may increase the risk of spreading scrub typhus from rats to humans. Chigger infestation on N. fulvescens varied significantly in different environments. The species abundance distribution showed a log-normal distribution pattern. The estimated number of chigger species on N. fulvescens was 126 species.
Introduction
Chiggers are the larval stage of mites from the families Trombiculidae and Leeuwenhoekiidae (Order Trombidiformes), which represent a small group of arthropods [1,2]. Trombiculid mites show 7 life cycle stages, and only those at the larval stage (chiggers) are ectoparasites living on rodents and other animals [3,4]. Chiggers are the only known vector of Orientia tsutsugamushi, the causative pathogen of scrub typhus. Scrub typhus is a febrile zoonotic disease, and Southwest China is an important focus of this condition [5–7]. The chestnut white-bellied rat Niviventer fulvescens (Gray, 1847), also known as the Indochinese white-bellied rat, is a common rodent species widely distributed in China, Northern Pakistan, Northwest and Northeast India, Nepal, Bhutan, Myanmar, Northern Vietnam, and likely also Thailand [8]. This rat species is an important agricultural pest and a common reservoir of several zoonotic diseases, including scrub typhus, hemorrhagic fever with renal syndrome, bartonellosis, and leptospirosis [9–11]. Although N. fulvescens is of medical importance, few scholars have studied the model of chigger infestation patterns on this species. Here, we retrospectively assessed the disease vector occurrence and ecological characteristics of chiggers living on N. fulvescens in Southwest China. This study sheds light on the population dynamics of N. fulvescens rats and their ectoparasites, including chiggers, thereby providing more detailed information on the surveillance of vector-borne diseases in Southwest China.
Materials and Methods
Ethics statement
The capture of animals including chestnut white-bellied rat was approved under the authorization of local wildlife authorities, and the use of animal hosts for research was reviewed and approved by the Animal Ethics Committee of Dali University. The approval number of animal ethics is DLDXLL2020-1104. Representative chigger mite samples and their animal hosts are deposited at the specimen repository of the Institute of Pathogens and Vectors of Dali University.
Collection and identification of chiggers and their hosts
We collected rodents and other small mammals (shrews, tree shrews, etc.) at 91 sampling sites distributed in 5 administrative regions, i.e., Yunnan Province, Guizhou Province, Sichuan Province, Chongqing Municipality, and Xizang Autonomous Region (Tibet), Southwest China (Supplementary Table S1; Supplementary Fig. S1). Animal hosts were collected using live traps (Guixi Mousetrap Apparatus Factory, Guixi, Jiangxi, China) placed in various habitats at each sampling site during the late afternoon and checked on the next morning. After being anesthetized with ether, trapped animal hosts were placed in a white tray (51 cm×36 cm), and chiggers were collected using a curette or lancet. Subsequently, chiggers were placed in 70% ethanol. The animal hosts were identified according to taxonomic reference data in the literature [8,12,13]. Chiggers were mounted onto glass slides prepared using Hoyer’s medium and identified at the species level under an Olympus CX31 trinocular microscope (Olympus Corporation, Tokyo, Japan) [4,14]. Based on the identification, chigger mites on N. fulvescens were selected as the targets of the present study.
Statistical analyses
In the present study, some conventional methods were used in statistics calculations, and the formulas omitted can refer to relevant references. To calculate the infestation of chiggers on N. fulvescens, the constituent ratio (Cr), prevalence (PM), mean abundance (MA), and mean intensity (MI) indices were estimated [15,16]. To calculate the structural characteristics of the chigger community, the species richness (S), Shannon–Wiener’s diversity index (H′), Pielou’s evenness (E), and Simpson’s dominance index (D) were also calculated [16–18]. To analyze the spatial distribution pattern of dominant chigger species among different individuals of N. fulvescens, the Cassie (CA), clumping (I), K, and patchiness (m*/m) indices were estimated [16,19,20].
In a semi-logarithmic rectangular coordinate system, the X-axis scaled with log intervals (log3N) included chigger individuals, and the Y-axis with arithmetic scales included chigger species. Preston’s log-normal model was used to fit the theoretical curve of species abundance distribution [16,21]. The expected total number of chigger species (ST) on N. fulvescens was estimated using the method based on Preston’s log-normal model [21,22].
In the above equation, Ŝ(R)=the number of theoretical species at the R-th log interval, S(R)=the number of actual chigger species at the R-th log interval, R=log interval R, R0= the mode log interval, S0=the number of chigger species at R0 log interval, β=the spread constant of distribution, n=the number of log intervals, and ST=the estimated number of chigger species on N. fulvescens. The β value was determined according to the best coefficient of determination (R2).
Results
Collection and chigger identification on Niviventer fulvescens
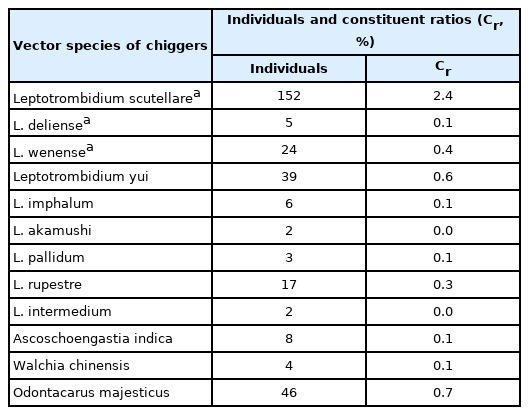
A total of 342 chestnut white-bellied rats were collected in 25 out of 91 (27.5%) sampling sites (Supplementary Table S1, S2; Supplementary Fig. S1). A total of 6,557 chiggers were identified in 136 N. fulvescens rats collected in 16 out of 25 (64.0%) sampling sites, and 6,384 (97.4%) of them were categorized as members of 100 different species belonging to 13 genera and 2 families (Supplementary Tables S2, S3). The remaining 173 (2.6%) chiggers remained unidentified due to 1) the absence of key characters (broken body), 2) key characters not clear due to debris, or 3) suspected new species. Among 100 chigger mite species identified on N. fulvescens, three and nine are the main and potential vectors of scrub typhus in China, respectively (Table 1). The 3 main vectors of scrub typhus were Leptotrombidium scutellare, Leptotrombidium wenense, and Leptotrombidium deliense, which accounted for 2.4% (152/6,384), 0.4% (24/6,384), and 0.1% (5/6,384) of the total (6,384) identified chiggers, respectively (Table 1).
Overall infestation and community structure of chiggers on N. fulvescens
The chiggers collected on 136 out of 342 N. fulvescens rats showed an overall prevalence of PM=39.8% (136/342), an overall MA=19.2 mites/per rat, (6,557/342), and an overall MI= 48.2 mites/per rat (6,557/136). The chigger community structure showed a species richness S=100, Shannon–Wiener’s diversity index H′=3.0, Pielou’s evenness index E=0.7, and Simpson’s dominance index D=0.1. The unidentified chiggers (173) were not included when calculating community estimation indices.
Infestation parameters of chigger species on N. fulvescens
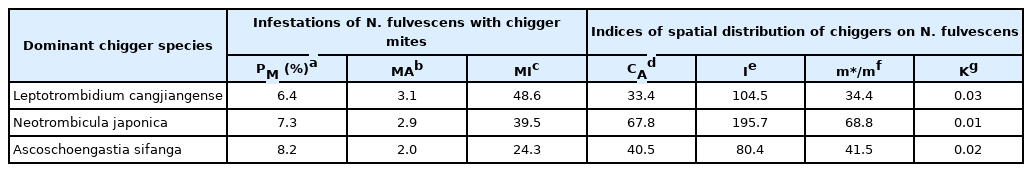
Leptotrombidium cangjiangense, Neotrombicula japonica, and Ascoschoengastia sifanga were the most dominant chigger species on N. fulvescens, with an overall Cr of 42.9% (2,736/6,384). L. cangjiangense was most frequently collected (Cr=16.8%, 1,070/6,384), followed by N. japonica (Cr=15.5%, 987/6,384) and A. sifanga (Cr=10.6%, 679/6,384). The prevalence value of A. sifanga (PM=8.2%) was slightly higher than that in both N. japonica (PM=7.3%) and L. cangjiangense (PM=6.4%). The MA and MI of L. cangjiangense were MA=3.1 (P>0.05) and MI=48.6 (P>0.05), respectively, showing the highest values compared to other chigger species (Table 2). The estimated CA, I, K, and m*/m indexes for the 3 dominant chigger species were higher than boundary values of determining aggregated distribution, i.e., CA, I, and K indices were higher than 0, while the m*/m index was higher than 1. Therefore, dominant chigger species exhibited an aggregated distribution among different N. fulvescens rats (Table 2).
Variability in the infestation of N. fulvescens rats with chiggers under different environmental conditions
We observed that the infestation of N. fulvescens rats with chiggers varied significantly depending on altitude. Most chiggers were identified on N. fulvescens hosts living at altitudes higher than 2,000 m above sea level, with a Cr of 82.6% (5,415/6,557), a value markedly higher than those at altitudes lower than 1,500 m (Cr=7.2%) and between 1,500 and 2,000 m (Cr=10.2%). All infestation indices of N. fulvescens with chiggers in altitudes over 2,000 m (PM=71.1%, MA=55.8, MI=78.5) were significantly higher than those at latitudes lower than 1,500 m and between 1,500 and 2,000 m (P<0.001, Table 3).

Infestation variations of chiggers on N. fulvescens at different altitudes in Southwest China (2001–2019)
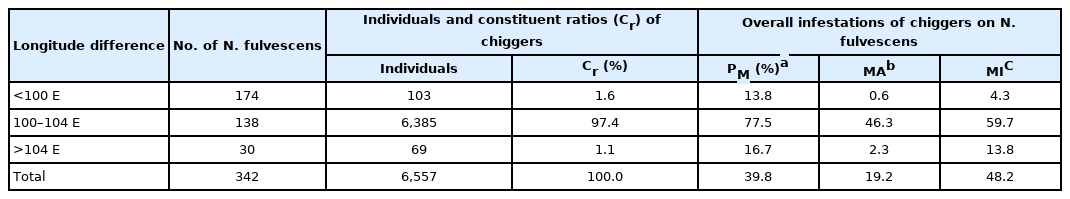
The infestation of N. fulvescens rats also varied significantly depending on latitude and longitude. A total of 4,221 chiggers were collected in 42 N. fulvescens rats collected at a latitude lower than 28°N, and the Cr rate of chiggers identified in rats collected at a latitude higher than 28°N was 64.4%, a value considerably higher than those at latitudes lower than 24°N, 24–26°N and 26–28°N. The infestation indices of N. fulvescens with chiggers at a latitude higher than 28°N were PM=82.4%, MA=82.8, and MI=100.5, and these values were significantly higher than those at latitudes lower than 24°N, 24–26°N, and 26–28°N (P< 0.001, Table 4). 138 N. fulvescens rats captured at a longitude between 100°E and 104°E, 107 were infested with 6,385 chiggers. The Cr rate of chiggers identified in rats collected at a longitude between 100°E and 104°E was 97.4%, a value markedly higher than those at longitudes lower than 100°E and over 104°E. The infestation indices of chiggers on N. fulvescens at a longitude between 100°E and 104°E (PM=77.5%, MA=46.3, MI=59.7) were significantly higher than those at longitudes lower than 100°E and over 104°E (P<0.001, Table 5).
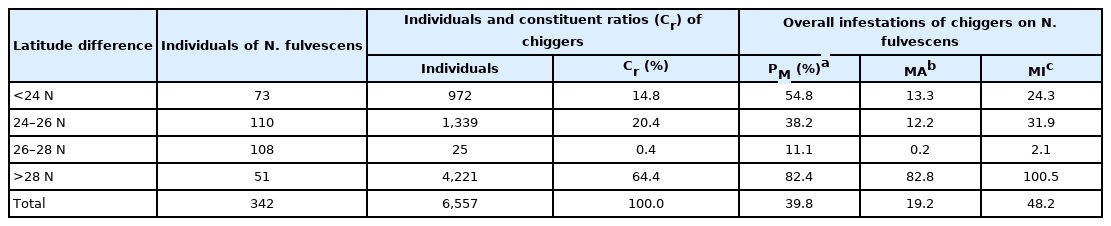
Infestation variations of chiggers on N. fulvescens at different latitudes in Southwest China (2001–2019)
Species abundance distribution of chigger mites on N. fulvescens
In intervals on a logarithmic scale, 19 chigger species with between 0 and 1 individuals on N. fulvescens appeared in log-interval 0, and 27 chigger species with between 2 and 4 individuals on N. fulvescens appeared in log-interval 1 These uncommonly sampled mites accounted for 46% (46/100) of the chigger species (Table 6). The species abundance distributions of the chigger community on N. fulvescens were successfully fitted with the Preston log-normal distribution model by using the equation Ŝ(R)=27e−[0.38(R−1)]2 (β=0.38, R2=0.92), and the theoretical curve showed a gradual downward trend at logarithmic intervals from 1 to 6 (Table 6; Fig. 1).

Theoretical curve fitting for the species abundance distribution of chigger community on N. fulvescens in Southwest China (2001–2019)
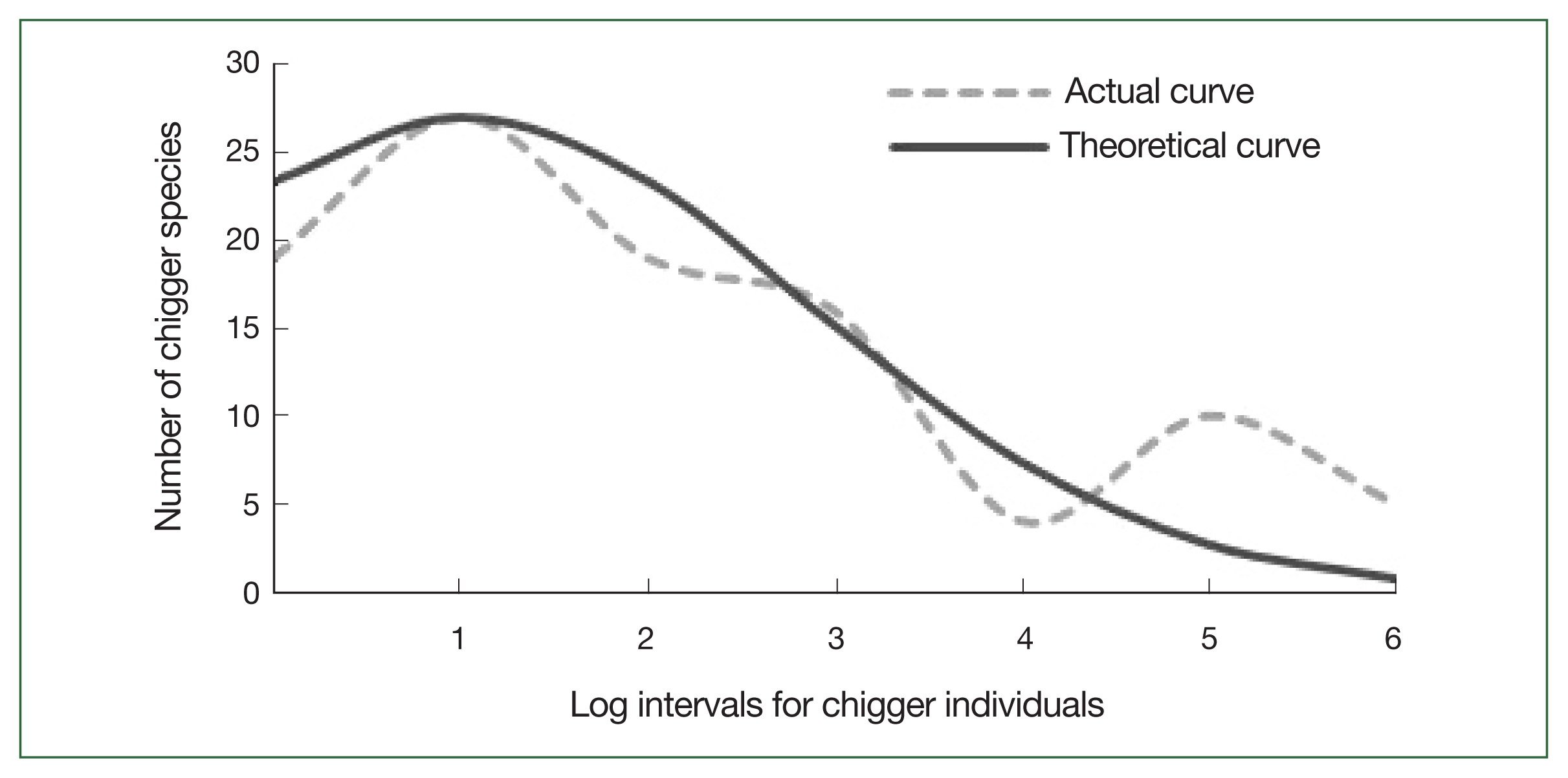
Theoretical curve fitting for the species abundance distribution of the chigger community on Niviventer fulvescens in Southwest China during the sampling period between 2001 and 2019.
The theoretical curve of species abundance distribution estimated a total of 126 species chigger species on N. fulvescens in Southwest China (ST=126), i.e., 26 species more than those identified by the sampling procedure (S=100).
Discussion
The chestnut white-bellied rat N. fulvescens is a common wild rodent in China more frequently found inhabiting outdoor sylvatic habitats (i.e., tropical forests, grasses, bushlands, etc.) than in indoor environments and whose distribution has been mainly associated with the occurrence of several sylvatic zoonoses [8–11]. In Southwest China, N. fulvescens often coexist with three sibling species, namely, N. fengi, N. huang, and N. mekongis [12]. In the present study, N. fulvescens individuals were differentiated from other species of the genus by using morphological characteristics, particularly dorsal hairs, ventral tails, teeth, skull structure, and some other measurements [8,12].
The chestnut white-bellied rat is widely distributed in Southwest China, although it is not the dominant rodent species in this region. For example, we captured 342 N. fulvescens, 1,981 Chevrieri’s (Apodemus chevrieri), and 715 striped (Apodemus agrarius) field mice in the same region [17,23]. N. fulvescens can be infected by many different chigger species and therefore exhibit a high community diversity index of chigger species. We found that species richness and infestation indices of chiggers on N. fulvescens are markedly higher than those on A. agrarius (S=14, PM=3.40%, MA=0.36 mites/per mouse, MI=10.63 mites/per mouse) [23]. Although we found that chigger mite species richness on N. fulvescens (S= 100) is lower than on A. chevrieri (S=107), all infestation indices of chiggers on N. fulvescens are higher than those on A. chevrieri (PM=31.95%, MA=6.32, MI=19.77) in the sampling region (Southwest China) [17]. These results suggest that N. fulvescens is highly susceptible to chigger infestation and is a major reservoir of chigger species in Southwest China.
In China, 6 main and more than 10 potential vector species of scrub typhus have been identified. These main vector species include L. deliense (Walch, 1922), L. scutellare (Nagayo et al., 1921), L. wenense (Wu et al., 1982), also referred to as L. kaohuensis or L. gaohuensis in the Chinese literature, L. rubellum (Wang and Liao, 1984), L. sialkotense (Vercammen-Grandjean and Langston, 1976), also referred to as L. jishoum in the Chinese literature, and L. insulare (Wei et al., 1989) [24–28]. Here, we identified 100 chigger species, including L. scutellare, L. wenense, and L. deliense, three of these 6 main vectors of scrub typhus in China, and 9 potential vectors (Table 1). The infestation of N. fulvescens may increase the risk of transmission of O. tsutsugamushi, the pathogen of scrub typhus that causes scrub typhus, from rats to humans.
We found that dominant chigger species (i.e., L. cangjiangense, N. japonica, and A. sifanga) accounted for 42.9% of the 100 chiggers identified on N. fulvescens. In contrast, Neotrombicula japonica (Tanaka et al., 1930) is a trombiculid mite that can occasionally bite humans, causing a type of dermatitis known as trombiculiasis or trombiculosis, and it has also been suspected as a possible vector of scrub typhus [3,29,30]. We found a high Cr of N. japonica on N. fulvescens, thereby potentially increasing the risk of infection and transmission of O. tsutsugamushi to humans [3,29,30]. Moreover, the medical significance of the dominance of L. cangjiangense and A. sifanga on N. fulvescens is unclear since there is no evidence that they can transmit O. tsutsugamushi.
The aggregated distribution of chigger species on N. fulvescens revealed a distribution pattern of chiggers among different hosts of the same species, which is consistent with previous reports [17,23]. In chiggers, as well as in some other ectoparasites, an aggregated distribution pattern may be considered beneficial for different life history traits, including survival, spread, mating, reproduction, and defense [3,17,31]. In suitable environments inhabited by potential hosts, chigger species often show a distribution pattern known as “mite islands” [3,31]. Given the current knowledge of the ecological characteristics of chigger communities, we cannot determine whether mite islands may or not influence the aggregated distribution pattern of chigger species among hosts living in different areas.
The results of the present study revealed high variability in the degree of infestation of chiggers on N. fulvescens rats living under different environmental conditions. Previous studies indicated that species composition and infestation characteristics of ectoparasites (including chiggers) in the same host species show frequent variation in heterogeneous regions showing different altitudes and latitudes [17,32], which is also consistent with our results [17,32]. The low host specificity of chiggers reported in the literature may partially explain why the same host species can be infected with different numbers of chiggers in different environments [17,23]. Parameters such as vegetation, temperature, humidity, and rainfall often show variation between regions with different altitudes and latitudes and may influence chigger populations [17,32]. Therefore, the variability in the degree of infestation of chiggers in individuals of the same host species may reflect the effects of environmental factors in different environments.
Species abundance distribution can successfully be used for describing the relationships between species and individuals in a given community [16,33]. The expected number of species in a community can be estimated by drawing the species abundance distribution curve [16,33]. The species abundance distribution of the chigger community on N. fulvescens showed a log-normal distribution pattern (Fig. 1), which is consistent with previous reports [16,17,33]. Our results reveal that the chigger community infecting N. fulvescens rats mainly consists of rare species, while dominant species are scarce.
In ecology, several methods to estimate the expected number of species in a community are available, and Preston’s log-normal distribution model is one of them [17]. This model estimated the occurrence of 126 chigger species (ST=126) living on N. fulvescens in Southwest China, i.e., 26 species more than the number of species identified by the sampling procedure (S=100). This result suggests that likely uncommon chigger species have been missed during sampling. It is almost unavoidable to miss rare species in the sampling procedure [15,17]. Further studies encompassing the complete distribution range of N. fulvescens and a significantly high number of collected samples are needed to identify all rare chigger species living on the host surface [17,34].
Supplementary Information
Acknowledgments
We are very grateful to the following people who contributed to our investigation both in the field and laboratory: Yun-Ji Zou, Zong-Yang Luo, Qiao-Hua Wang, Yong Zhang, Cong-Hua Gao, Nan Zhao, Jian-Chang He, Guo-Li Li, Yan-Liu Li, Xue-Song He, Chang-Ji Pu, Ji-Wei Guo, De-Cai Ouyang, Shuang-Lin Wang, Xing-Shun Zhu, He Sha, Long Zhou, A-Si Di, Cheng-Wei He, some colleagues, and college students. We are also grateful to the Expert work station for Dao-Chao Jin in Dali Prefecture. This work was supported by the Major Science and Technique Programs from Yunnan Province (Grant No. 202102AA310055-X) and the National Natural Science Foundation of China (No. 82160400).
Notes
Author contributions
Data curation: Chen YL
Formal analysis: Chen YL
Funding acquisition: Guo XG
Investigation: Song WY, Ren TG, Fan R, Zhao ZW, Dong WG
Methodology: Chen YL, Zhang L, Huang XB
Project administration: Guo XG
Software: Chen YL
Supervision: Guo XG, Jin DC
Visualization: Chen YL
Writing – original draft: Chen YL
Writing – review & editing: Chen YL, Guo XG
The authors have no conflict of interests.