Four cases of gastric submucosal mass suspected as anisakiasis
Article information
Abstract
Anisakiasis is a parasitic disease caused by ingestion of raw fish infected with anisakid larvae. Endoscopic changing patterns of submucosal lesions in chronic gastric anisakiasis have not been known yet. Here we report 4 cases of suspected gastric anisakiasis which were improved during follow-up periods without surgical treatment. The patients presented with abdominal pain, nausea and vomiting after consuming raw marine fish, and visited our gastroenterology outpatient department. Their endoscopic findings showed firm and yellowish submucosal masses accompanied with eccentric erosions. Histologic findings showed severe eosinophilic infiltrations. In blood tests, peripheral eosinophil counts and total IgE levels were elevated. We believed that all cases were caused by larval anisakid infections. The submucosal mass lesions disappeared during the follow-up periods of 2 to 4 mo.
INTRODUCTION
Anisakiasis is a parasitic disease caused by the consumption of raw fish infected with anisakid larvae. The first case of anisakiasis was reported in the Netherlands (Van Thiel et al., 1960). Thereafter, many cases have been reported in Japan, Korea, the Netherlands and in western Europe in regions where raw fish are consumed. In Korea, a removal of Anisakis larva from the oropharynx was reported as the first case in 1971 (Kim et al., 1971). Thereafter, many anisakiasis cases have been reported via gastroduodenoscopy (Lee et al., 1981; Shim et al., 1995).
Acute gastric anisakiasis is diagnosed by visualization of anisakid larvae with endoscopy. Whereas chronic anisakiasis is diagnosed by observation of anisakid larvae embedded in pathology specimens obtained during surgery. Basically, anisakiasis can not be diagnosed if anisakid larvae are not discovered. Moreover, there has not been known effective chemotherapeutic treatment for chronic anisakiasis.
The present authors describe suspected cases of chronic anisakiasis representing as submucosal tumor-like lesions in the stomach, and observed the changing patterns of submucosal lesions by endoscopic follow-up.
CASE RECORD
Case 1
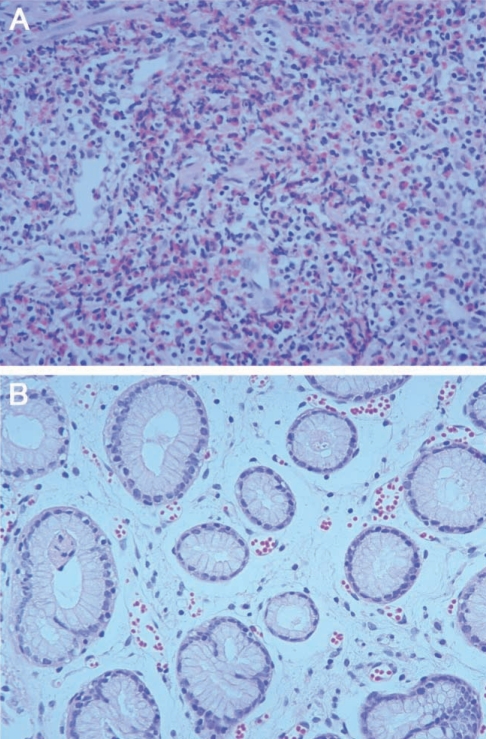
A 51-year-old man visited our outpatient department. He complained of nausea and epigastric pain which had sustained 3 to 4 days before admission, and aggravated from 24 hr before visit. He had no specific relevant history of hypertension, diabetes or allergic diseases. Physical examination revealed soft abdomen, mildly tender epigastrium but no rebound tenderness and abnormal mass-like lesions. We prescribed antacids and mucosal protecting agents for symptomatic care. Gastric endoscopy was performed 5 days after presentation. Endoscopy revealed a 2 cm sized submucosal tumor-like lesion with turtle back-like change, at the antrum of the stomach. Two similar lesions were found at the antrum and greater curvature (Figs. 1A, 1B). A biopsy revealed severe eosinophilic infiltrations and inflammation (Fig. 2A). History taking revealed eating of raw anchovy before the symptom onset. Considering the history of eating raw fish, the symptom pattern, endoscopic and histopathologic findings, we suspected the submucosal tumor-like lesion as being caused by anisakiasis and prescribed albendzole. Two months later, follow-up examination revealed that the submucosal tumors at the antrum reduced in size (Figs. 1C, 1D), and eosinophilic infiltrations in the biopsy specimen were remarkably reduced (Fig. 2B). Laboratory data showed white blood cell count of 7,700 × 103/µL, eosinophils 4.3% and total IgE of 439 IU/ml. Two mo later, endoscopy revealed a further reduction in the size of the submucosal tumor-like lesions (Figs. 1E, 1F).
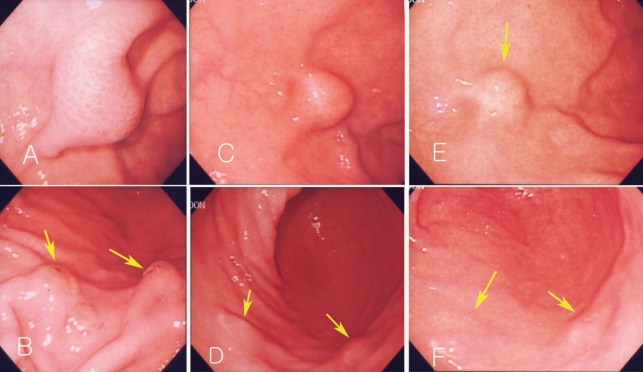
Endoscopic findings of Case 1. Endoscopic gastroduodenoscopy shows a submucosal tumor-like lesion in the fundus of the dome of the stomach (2 × 2.5 cm in size) (A). Multiple submucosal tumor-like lesions with erosions are seen in the antrum (arrow) (B). The size of the same submucosal mass-like lesion decreased after 2 mo (C, D) and 4 mo (E, F).
Case 2
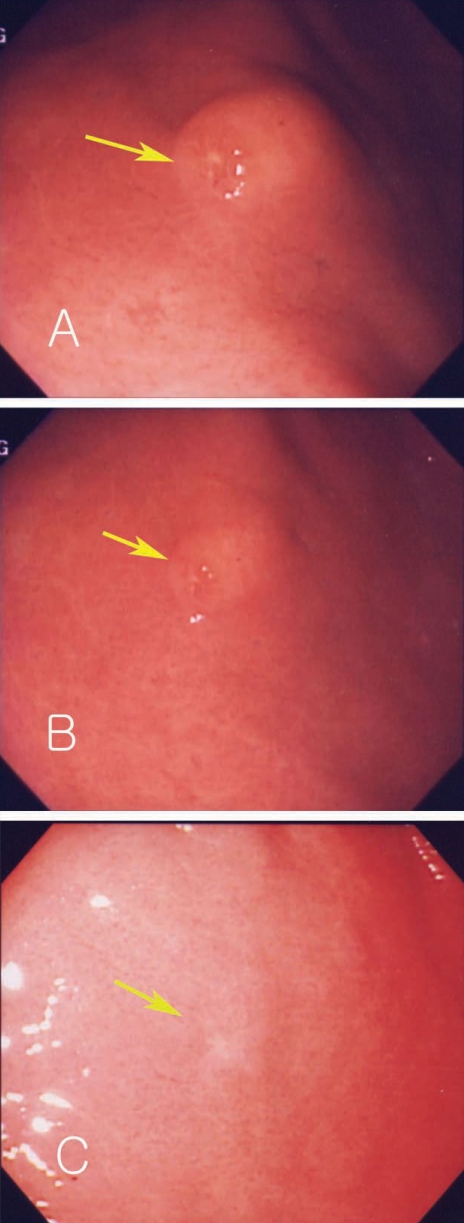
A 45-year-old man complained of abdominal pain and loose stools which had persisted 3 to 4 times a day but without bloody or mucoid diarrhea, fever, or myalgia. His medical history showed no evidence of any specific disease. Physical examination showed stable vital signs. Abdominal epigastric tenderness was presented without rebound tenderness. We prescribed antacids and mucosal protecting agents for conservative management and gastric endoscopy was performed. Endoscopy showed a submucosal tumor-like lesion with yellowish surface at the proximal part of the antrum in the stomach. Some lesions showed erosions and hard surface consistency (Fig. 3A). A biopsy finding revealed severe gastritis with eosinophilic infiltrations. History taking revealed eating of raw sea eels and ascidians before the symptom onset. Laboratory data showed white blood cell count of 13,920 × 103/µL, and eosinophils 12.8%. Considering his history, laboratory test results, and endoscopic and pathologic findings, a submucosal tumor-like lesion caused by anisakid larval infection was suspected. We prescribed albendazole and 2 months later performed a follow-up endoscopy which showed a decrease in size and scarring corresponding to the location of the previous submucosal lesion at the anterior wall of the antrum (Figs. 3B, 3C). A biopsy showed reduced eosinophilic infiltrations and laboratory test showed that white blood cell counts reduced to 7,700 × 103/µL, with eosinophils at 9.6%.
Case 3
A 56-year-old woman visited our outpatient department, complaining of nausea and vomiting, which occurred 1 week previously. She had received medication at local clinic for several days. Endoscopy showed a submucosal tumor-like lesion at lesser curvature of the stomach and a biopsy showed chronic gastritis with severe eosinophilic infiltrations. She had eaten a raw flatfish 2 days ago. In laboratory tests, her white blood cell count was 6,530 × 103/µL, with eosinophils 9.9%, and her total IgE was more than 2,000 IU/ml. We suspected gastric anisakiasis, and prescribed albendazole. Two mo later, gastric endoscopy was performed and showed reduced mucosal erosion at the lower body of the stomach and a decrease in size of the submucosal lesion. A biopsy showed a reduced eosinophilic infiltration, and laboratory test showed white blood cell count of 5,490 × 103/µL and eosinophils 3%. Five mo after presentation, a second follow-up endoscopy revealed that the submucosal lesion disappeared. At this time, her white blood cell count was 3,900 × 103/µL, with eosinophils 3%.
Case 4
A 52-year-old woman complained of epigastric pain from 3 to 4 days before admission. Several days before she consumed raw sea eels and took a pain-killer. Endoscopy was performed 6 days after the symptom onset, and showed erosion, ulceration, and a submucosal elevated lesion at the antrum of the stomach. The CLO test was positive, and a biopsy showed chronic gastritis with eosinophilic infiltrations. Laboratory examination showed white blood cell count of 9,880 × 103/µL with eosinophils 9.2%. Clinically we suspected gastric anisakiasis, and prescribed medications only for symptomatic managements. Two months later, she was followed-up by endoscopy which showed scarring at the previous erosion. Biopsy showed reduced eosinophilic infiltrations. Gastric anisakiasis was suspected and albendazole was prescribed.
DISCUSSION
Histologic findings in chronic anisakiasis can be classified into 4 types according to the duration of infection and the degree of larval denaturalization (Kojima, 1966). The first type is phlegmon type wherein larvae are located in the submucosal layer with eosinophil, neutrophil and histiocyte infiltrations. The second is chronic abscess type. Larvae are denaturalized and abscess is formed by eosinophils and fibrin. The third type is abscess-granulomatous type. This type develops in 6 mo after anisakid larvae infection, and shows progressive granuloma and fibrosis. The fourth type is the granulomatous type where abscesses have become granulomas. All of our cases are considered, if they were anisakiasis, the phlegmon type or chronic abscess type.
The clinical symptoms of anisakiasis are classified in terms of acute and chronic infections (Alonso-Gomez et al., 2004). Acute infection occurs by invasion of the stomach or intestine by third stage larvae. These larvae are found in the mucosa or submucosa of the stomach or intestine. Clinical symptoms develop due to the physical effects of larval invasion into the mucosa of the stomach or because of hypersensitivity to the larvae or its secretions. The symptoms are characterized by epigastric pain (caused by a reaction to peptidases), nausea and vomiting (Dziekonska-Rynko et al., 1997). Chronic anisakiasis results from an invasion of larvae into the mucosa or submucosa, and causes abscesses or eosinophilic granulomas, with fever, eosinophilia, diarrhea, and abdominal pain. It can mimic appendicitis, gastroduodenal ulcer, inflammatory bowel diseases, or intestinal obstruction. Rarely, anisakid larvae invade the lungs, spleen, liver, or pancreas.
In acute gastric anisakiasis, epigastric pain is the most common symptom, especially within a day of consuming the responsible fish, and this may persist for 1 to 3 days. Endoscopy in acute anisakiasis shows edema, erosion, erythema, hyperemia, hemorrhage and/or ulcers. Among these endoscopic findings, edema and hemorrhage are most common. Various kinds of marine fishes, especially, yellow corvina, sea eel, ling and yellowtail are the sources of human infections (Im et al., 1995). In our cases, 3 patients complained of epigastric pain and other symptoms including nausea, vomiting and diarrhea, which developed within 1 day after eating raw fish.
Detailed history taking about eating raw marine fish is very important for diagnosis of gastric anisakiasis. Other diagnostic tests include gastroscopy and blood tests (Song et al., 1999). In our cases, all had a history of eating raw marine fish, and eosinophilia in blood test (Table 1). However, a precise diagnosis of acute gastric anisakiasis is possible only by discovery of anisakid larvae by gastroscopy or surgery (Lee et al., 1999; Ko et al., 1988; Lee et al., 1993). In gastroscopy, most of anisakid larvae are found alive (Yoon et al., 2004), in association with edema, and sometimes with hemorrhages (Seol et al., 1994). Biopsy shows severe eosinophilic infiltrations, and granulomatous changes consisting of eosinophils, neutrophils, lymphocytes, and histiocytes surrounding the larvae. In our cases, gastroscopy showed hard submucosal tumor-like lesions with eccentric erosions. Erosions are believed to represent invading sites of larvae, and in our cases erosions disappeared without scarring at follow-up gastroscopy. In addition, biopsies showed severe eosinophilic infiltrations, which were reduced at follow-up biopsies.
In the case of chronic anisakiasis, larvae are degenerated and absorbed, and endoscopic or histologic diagnosis is difficult. Recently immunologic methods have been reported (Castan et al., 2002). Specific IgE to Ani S 1, a major antigen purified from A. simplex larvae, was reported to be helpful for diagnosis of anisakiasis (Caballero et al., 2002). However, we could not apply this technique to our cases.
There are few effective chemotherapeutics against anisakiasis. However, albendazole was reported to be effective in experimental anisakiasis of guinea pigs (Dziekonska-Rynko et al., 2002). Effectiveness of albendazole medication was also reported in human anisakiasis (Moore et al., 2002). Therefore, we prescribed albendazole (400 mg) twice daily for 14 days between day 13 to day 126 after raw fish intake. During the follow-up period, clinical symptoms were improved, and endoscopy showed decreased, or disappearance of submucosal lesions, and in addition, biopsies showed reduced eosinophilic infiltrations. However, the efficacy of albendazole medication requires further verification in man and experimental animals.
There are shortcomings in our report. Most of all, we couldn't confirm anisakiasis. But there are several reasons why we suspected our cases as anisakiasis. First, most patients had history of eating raw fish which are known as carriers of anisakiasis. Second, our patients had acute symptoms such as epigastric pain, nausea and vomiting which were non-specific but could be caused by gastric anisakiasis. Third, endoscopic biopsy showed granulomas associated with eosinophilic infiltrations which could be an important clue for suspecting a parasitic infection. Fourth, in follow-up period, any other diseases, such as gastric malignancy, peptic ulcer diseases and other parasitic infections were not discovered. Fifth, improvement of eosinophilic infiltration was shown in follow-up endoscopic biopsies.