Seroprevalence of toxoplasmosis in Korean pregnant women
Article information
Abstract
This study was performed in order to evaluate the sero-epidemiological status of toxoplasmosis in pregnant Korean women. Among 5,175 sera and 750 amniotic fluid samples obtained from pregnant women, 41 serum samples (0.79%) and 10 (1.33%) amniotic fluid samples tested positive for IgG antibodies by ELISA. Fifty one cases showing a score more than 0.25 on ELISA were tested for PCR reaction against the SAG1 gene. Only one case of the 51 ELISA positive cases exhibited a positive reaction on all tests. This case had a history of acute nephropyelitis during early pregnancy, but fortunately, had delivered a phenotypically healthy baby. In this study, the seroprevalence of toxoplasmosis in pregnant women was found to be comparatively low, consistent with previous reports from Korea. However our trials, performed with a variety of diagnostic tools, were considered to be useful for the precise diagnosis of congenital toxoplasmosis.
The apicomplexan parasite, Toxoplasma gondii, is a globally distributed obligate protozoan, which can infect the central nervous system of warm-blooded animals, including humans. Toxoplasmosis is normally asymptomatic in immunocompetent individuals. However, T. gondii infection, when acquired during pregnancy, can lead to fetal infection, which may ultimately result in the loss of the fetus, or in lesions which normally involve the brain and eyes (Koppe et al., 1986). The risk of maternal fetal transmission of infection increases with gestational age at the time of exposure, whereas the incidence of severe disease decreases (Antoniou et al., 2004). The frequency of severe congenital infections can be limited by the early screening of pregnant women (Florence et al., 1999). Prevention of congenital toxoplasmosis in pregnant women has been based mainly on serological tests for anti-Toxoplasma antibodies. Many serological tests, including the haemagglutination test, latex agglutination test (LAT), ELISA, and indirect fluorescence antibody test, have been utilized in the detection of antibodies against T. gondii. There have been several reports regarding the screening of anti-T. gondii antibodies among Koreans. Ryu et al. (1996) demonstrated 4.3% and 0.94% of positive rates, using ELISA and LAT kits among pregnant women who visited medical institutes in Yangpyong-gun and Kwangju-gun of Kyonggi-do. However, infection should be diagnosed at the early acute stage, when treatment is more effective.
This study was performed in order to determine the levels of anti-Toxoplasma antibodies in pregnant women using ELISA and to detect the early infection using PCR.
A total of 5,175 sera and 750 amniotic fluid samples were tested, all of which had been obtained from pregnant women transferred for prenatal diagnoses at the Kangnam St. Mary's Hospital (Department of Obstetrics and Gynecology). The sera and amniotic fluids were frozen at -20℃ until use. The age range of the pregnant women was 20-40 years. The crude extract was prepared from the collected tachyzoites of the RH strain of T. gondii. Antigen preparation was performed according to the methods described by Choi et al. (1992). ELISA was performed by a modified version of the methods of Choi et al. (1992). Absorbance was tested at 490 nm with a 96-well plate reader (Elx 800, Bio-Tek Instruments, USA). The cut-off value was determined by a modified version of the methods of Choi et al. (1992). Two hundred randomly selected sera were subjected to indirect latex agglutination tests (ILA), and the cut-off absorbance for a positive ELISA reaction was determined to be 0.25, the mean absorbance of the ILA 1:32 titer group. SAG1 (p30) gene targets were amplified for PCR. A published sequence of 689 to 895 bp of the p30 gene (Burg et al., 1988) was amplified with primer 5'-AGC TGG TGG ACG GGG GGA TTC-3' and 5'- TCT GCA CCG TAG GAG CAC C-3'. The amniotic fluid samples of the seropositive women were centrifuged at 2,000 g for 10 min, and the supernatants were discarded. The DNA was extracted from the pellet by a technique adapted from the method described by Loparev et al. (1991). The pellets were suspended in lysis buffer containing 10 mM Tris-HCl, 0.1 mM EDTA, 0.15 M NaCl, 0.5% Triton X-100, 0.5% sodium dodecyl sulfate, and 5 mg/ml of proteinase K; the extraction procedure was performed according to the generally-accepted protocol (Maniatis et al., 1982) and included two purification steps involving phenol-chloroform-isoamyl alcohol (25/24/1). The DNA was initially precipitated with cold ethanol. After centrifugation, the DNA pellets were washed in 70% ethanol, centrifuged, then resuspended in sterile water. The DNA concentrations were determined by spectroscopy (Pharmacia Biotech, Saint-Quentin en Yvelines, France), and 1 µg of DNA was used to perform PCR. Each sample was amplified and duplicated with primers in a final volume of 50 µl, containing 10 mM of dNTPs, 20 pM of each primer, and 1.25 U of Tag polymerase. The reaction was performed for 35 amplification cycles, each consisting of 1 min at 94℃, 1 min at 55℃, and 1 min at 72℃. The PCR products were then separated on a 2% agarose gel, and the amplified bands were compared to the bands obtained with the positive T. gondii DNA controls. Statistical comparisons were made with χ2 tests (P < 0.05).
The serological positive rate of toxoplasmosis in the 5,725 pregnant women was 0.88% (200 cases were duplicated). Forty one (0.79%) out of 5,175 sera and 10 (1.3%) out of 750 amniotic fluid samples from pregnant women were determined to be positive with ELISA (Table 1). In the previous study, Choi et al. (1985) studied the rates of toxoplasmosis prevalence. They screened 377 pregnant women and 43 pelvic tumor patients at St. Mary's Hospital, and reported a positive rate of 0.5% in the former and a 7.0% positive rate in the latter. One case out of the 51 seropositive cases exhibited a positive PCR reaction to the SAG1 gene (Fig. 1). This patient had a history of acute nephropyelitis during early pregnancy, but had fortunately delivered a phenotypically normal baby. Other 50 seropositive cases may acquire Toxoplasma infection before the present pregnancy.
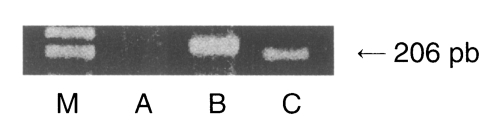
Result of PCR amplification of the p30 gene of the genomic DNA from amniotic fluids (AF) of seropositive pregnant women. M, DNA marker; A, negative control (normal AF); B, positive control (RH strain of Toxoplasma gondii), and C, patient (who had a history of acute nephropyelitis during early pregnancy).
Although the PCR technique employed in this study could detect as few as 1 to 10 parasites per sample, a sensitivity similar to that associated with other PCR methods (Robert-Gangneux et al., 1999), these false positive results may be attributable to a low fetal parasitic burden (the infant was asymptomatic at birth). We found no obvious clinical characteristics, which are unique or specific to toxoplasmosis. Indeed, many toxoplasmosis are asymptomatic, or exhibit symptoms which mimic other condition or are completely overlooked.
The seroprevalence of toxoplasmosis is known to increase with ages. In this study, we observed an increase in seropositivity which occurred directly with the mother's age. Im et al. (1991) emphasized that prenatal detection of antibodies against T. gondii in pregnant women is crucial with regard to the management of serious congenital complications, including abortion due to intrauterine fetal death, microcephalus and hydrocephaly. In this study, although we did not take into account past obstetric history, over 98% of the seronegative pregnant women were determined to be at risk of sero-conversion during pregnancy, and eating raw meat or feeding pet cats were the principal risk factors for Toxoplasma infection in pregnant women in Korea. Our study on anti-T. gondii antibody titers using amniotic fluid from pregnant women is the first screening of its kind in Korea.
