The effect of microfilament inhibitor on the Cryptosporidium infection in vitro
Article information
Abstract
This study was focused on the effects of microfilament inhibitor, Cytochalasin D (CD) on the invasiveness of sporozoites of Cryptosporidium spp. into the host cells. MDCK and AGS cell lines were used as host cells for C. parvum and C. muris, respectively. When MDCK cells were pretreated with CD for 1 hr before inoculation of the sporozoites, C. parvum infection was significantly inhibited when compared to the control cells. These inhibitory effects of CD on the rate of infection were dose-dependent. In addition, C. muris infection was hampered when AGS cell lines were pretreated with CD. However, the capability of invasiveness of the sporozoites into the host cells was not greatly influenced by the pretreatment of sporozoites with CD before infection. These results suggest that microfilaments of host cells, rather than parasites, play an important role for the invasion of Cryptosporidium spp.
INTRODUCTION
Genus Cryptosporidium is a well known coccidian protozoa which has a wide range of hosts. Although a lot of different names of the species have been reported, only six species, such as C. parvum, C. muris, C. baileyi, C. meleagridis, C. serpentis, and C. nasorum, are generally accepted as truly valid ones (O'Donoghue, 1995). The majority of human cryptosporidiosis has been reported in immunocompromised patients infected with C. parvum (Griffiths, 1998) and they usually suffer from a chronic, devastating illness refractory to all therapeutic modalities. However, there are two more species other than C. parvum that are known to infect human such as C. baileyi and C. muris (Ditrich et al., 1991; Katsumata et al., 2000).
C. parvum and C. muris have been reported to have a large amount of actin in their cytoplasm and pellicles all through their developmental stages (Yu and Chai, 1995; Yu, 1998) and the gene that encodes actin has been isolated in C. parvum (Kim et al., 1992). However, the function of actin in this organism has not been well defined yet. For the successful infection of coccidia, invasive zoites are the most important developmental stages. They are suspected to invade host cells via a capping phenomenon in Eimeria spp. and the parasite's contractile system is considered to be involved in this process (Russell, 1983). For the host cell invasion of Cryptosporidium, there are very little findings known until now. In this study we investigated the role of microfilament during invasion of Cryptosporidium to host cells using microfilament inhibitors.
MATERIALS AND METHODS
Oocyst preparation
Oocysts of C. parvum were collected and purified as described by Yu et al. (2000). The oocysts of C. muris were isolated from the naturally infected laboratory mice (C57BL/6) at the laboratory of Parasitology in Konkuk University in 1997, and have been maintained through in vivo cultivation using the same mouse (SPF) strain. The oocyst collection and purification methods are the same as those of C. parvum. But mice were not immunosuppressed for the C. muris oocyst collection and the feces were collected from 20 to 40 days after oral infection.
Host cell culture and sporozoite inoculation
As host cell lines, Madin-Darby Canine kidney cell (MDCK) and human stomach adenocarcinoma (AGS) (Korean Cell Line Bank, Seoul, Korea) were used for C. parvum and C. muris, respectively. The cells were incubated in RPMI 1640 with L-glutamine (Sigma) containing 15 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid (HEPES), 14 mM sodium bicarbonate, 100 U of penicillin per ml, 100 µg of streptomycin per ml, and 0.25 µg of amphotericin B per ml. For a routine cell passage, 10% fetal bovine serum (FBS) (Gibco BRL, Gaithersburg, MD) was used, however, after sporozoite inoculation, 1% FBS was used for the MDCK cells and 10% FBS was used for AGS cells. The host cells for infection were plated onto coverglass 22 mm2 placed in 6 well plates and incubated at 37℃ in a 6% CO2-94% air humidified incubator. Oocysts were washed once with RPMI 1640 medium after excystation by incubating in 1% trypsin in PBS at 37℃ for 1 hr, and inoculated 4 × 106 sporozoites into subconfluenced monolayers per well.
Cytochalasin D treatment
A stock solution of cytochalasin D (CD; Sigma) was prepared by dissolving in dimethyl sulfoxide (DMSO; Sigma) and adjusting the concentration of CD to 100 µg/ml in RPMI 1640. This was stored at -20℃ and final DMSO concentrations were always under 0.05% (v/v). RPMI 1640 containing the desired concentrations of CD, 0.5, 1.0, 2.5, 4.0 or 5.5 µg/ml, were added to the monolayers. Controls were untreated monolayers and monolayers to which was added medium containing DMSO (0.05%) alone. The experiments were then divided into two groups. In one group, monolayers were preincubated with various concentrations of CD or DMSO for 1 hr before Cryptosporidium inoculation and the same concentrations of CD were also included in the media after infection. In the other group, sporozoites of Cryptosporidium were preincubated with various concentrations of CD or DMSO for 1 hr, and then challenged into monolayers which has not been exposed to CD. The media for monolayers did not contain CD after sporozoite inoculation.
Infection evaluation
The cover glass was taken out from the well after 24 hr of incubation at 37℃ in a 6% CO2-94% air humidified incubator, washed in PBS, pH 7.4 and fixed in methanol for 1 min. The cover glass was stained by Hematoxylin and Eosin and then mounted on the slide glass. The total number of parasites infected monolayers was counted from 25 fields under of a light microscope with a resolution of 1,000 × (oil) lens (Olympus). The experimental data of each group, repeated four times, were compared with those of control group. The significant difference between the groups was evaluated by Student's t-test.
RESULTS
Cytochalasin D effect on C. parvum infection in vitro
Cytochalasin D at the concentrations tested did not show any toxic effect on the MDCK cell monolayer within 24 h culture. The sporozoite of C. parvum invasion into MDCK cells was not affected by DMSO treatment (Fig. 1). The infected parasite number of C. parvum in MDCK cells significantly decreased (P < 0.05) compared to that of control group when the sporozoites were inoculated to host cells preincubated with CD (Fig. 1A). Although low concentrations of cytochalasin D had a little effect on the parasite infection, pretreatment of the cells with higher concentrations of CD (2.5 µg/ml) resulted in marked decrease of parasite burden in the host cells. Thus, the infected parasite number in the host cells pretreated with 2.5 µg/ml CD was about 10% of that of control group.
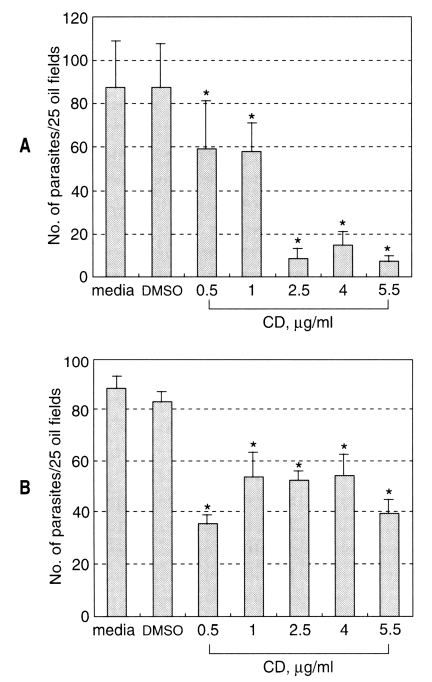
Effect of CD on the Cryptosporidium parvum infection into MDCK cells (mean ± SD). A, the host cells were pretreated with CD for 1 h before sporozoite inoculation; B, sporozoites were pretreated with CD for 1 hr before infection. *, p < 0.05.
When the MDCK cells were infected with sporozoites pretreated with CD, the invasion was slightly, but significantly (P < 0.05), inhibited at the tested concentrations (Fig. 1B). From these results, it seemed that the microfilament of MDCK cells were more important in the process of C. parvum infection.
Cytochalasin D effect on C. muris infection in vitro
Cytochalasin D at the concentrations tested did not show any toxic effect on the AGS cell monolayer within 24 h culture. The sporozoite of C. muris invasion into AGS cells was not affected by DMSO treatment (Fig. 2). The invasion of C. muris sporozoites on AGS cells pretreated with CD was significantly inhibited compared to that of control group (Fig. 2A). The infected parasite number in the host cells pretreated with 1 µg/ml CD was only 10% of that of control group. On the other hand, the sporozoite invasion was not significantly influenced by pretreatment of the sporozoites with CD (Fig 2B). These results suggest that the microfilament of AGS cells plays an essential role for successful infection of C. muris sporozoites.
DISCUSSION
The role of cytoskeletal proteins in coccidian parasite's invasion has been evaluated using inhibitors against microtubule or microfilament. Cytochalasin D is well known microfilament inhibitor that shows inhibitory effect by binding on the subunits of actin and actomyosin (Tannenbaum et al., 1977).
CD was proved not to affect the attachment of Toxoplasma gondii but to prevent the entry into peritoneal macrophages and into the bladder tumor 4934 cells (Ryning and Remington, 1978). In this paper, it was suggested that T. gondii acts on the nonphagocytic cell in some undefined way, inducing phagocytosis rather than utilizing the cell merely as a passive agent during the entry process. In other words, host cells actively participate in the process by which T. gondii gains entry to the cells. Additionally, in case of Eimeria species, it was implied that a microfilamentous system is active during a host cell invasion because the invasive behavior of E. acervulina and E. tenella was inhibited by cytochalasin B (Russell, 1983).
The cytoskeletal system is known to regulate the sporozoite motility as well, thus being an important factor for host cell invasion in T. gondii and Eimeria species (Schwartzman and Pfefferkorn, 1983; Russell and Sinden, 1981). The fact that the sporozoite motility of Eimeria was inhibited by treatment with cytochalasin B, but not by colchicine, vinblastine sulfate or other microtubule inhibitor, indicated that all aspects of motility are microfilament-based contractile system type.
There are several reports describing the role of cytoskeletal system in the process of C. parvum invasion into host cells. Wiest et al. (1993) reported that microtubule inhibitors induced 77% worm number reduction in C. parvum infection into human enterocyte cell line. This study showed that microtubules have an important role for the C. parvum invasion and may represent targets for development of new therapeutic drugs for treatment of cryptosporidiosis.
In the present study, pretreatment of host cells with CD inhibited Cryptosporidium species invasion into the host cells. Both C. parvum and C. muris infection were prevented with the disruption of host cells' microfilaments using CD. Therefore, the microfilament of the host cells may play an important role in Cryptosporidium entry by making host cells participate actively in the entry process, like in T. gondii or Eimeria spp. Another possible explanation could be that, in mammalian cell lines, the membrane receptors are known to be anchored by a subtending cytoskeleton and could act as a substratum for the parasite's contractile system to work against (Shelterline, 1980). Therefore, the disruption of microfilaments in the host cells may induce structural changes, causing the surface receptors to be masked or distorted during such process, which would inhibit sporozoite to recognize receptors for invasion into the cell. The membrane bound host cell receptors related to the cytoskeletal system may have a function in the initial process of Cryptosporidium invasion.
In this study, C. parvum invasion was partially, but significantly, inhibited when the sporozoites were pretreated with CD before infection. The effect of CD was known to be reversible within 60 min after removal of this agent (Ryning et al., 1978). However, since most sporozoites of Cryptosporidium invade host cells within 60 min, the time interval is considered to be enough to observe the inhibitory effect of CD on the sporozoites invasion. The result with sporozoites is somewhat different from previous studies reported by Forney et al. (1998). They described, although the sporozoite motility decreased with CD treatment, sporozoite's invasion into host cell was not affected. There also are some key points which could explain the discrepancies between the previous findings by Forney et al. (1998) and present study. One thing is that they did not disrupt the microfilament of host cells, so there was no description about the role of microfilament of host cells. Another one is that different cell lines, which might have different amounts and distribution of microfilaments, were used for C. parvum infection. In addition, different CD concentrations tried for both two studies may be responsible for different results between two studies.
In terms of C. muris infection, we found that, whereas the host cell microfilament disruption induced lower infection rates, the inhibition of the microfilament of parasite itself did not inhibit the parasite invasion. Since the effect of CD on the motility of C. muris sporozoite was not observed in this study, it is hard to determine the exact role of the parasite motility in the host cell invasion. The reason for using AGS cell lines as host cells in C. muris infection is due to the higher susceptibility of the AGS cells than the MDCK cells against C. muris infection (data not shown).
In conclusion, this study shows that intact host cell microfilament is required for the successful infection of Cryptosporidium species. Further investigation with respect to the interaction of microfilament between host cells and Cryptosporidium is needed for the full understanding of host-parasite relationship of this parasite.
