Excretory-secretory product of Paragonimus westermani newly excysted metacercariae inhibits superoxide production of granulocytes stimulated with IgG
Article information
Abstract
It is well known that the cysteine proteases in excretory-secretory product (ESP) of Paragonimus westermani newly excysted metacercariae (PwNEM) are capable of degrading IgG in vitro. Recent evidence suggests that the IgG-coated surface, such as found on parasites, is one of the most effective physiologic stimuli for granulocyte activation. Therefore, this study was designed to investigate the effect of excretory-secretory product (ESP) of PwNEM on superoxide production of granulocytes stimulated with IgG. The 96-well plates were coated with human IgG (0, 10, 30, 100 µg/ml) in the absence or presence of ESP. When granulocytes were incubated in the wells coated with human IgG in the presence of ESP, the level of superoxide production of granulocytes was reduced to about 90% when compared to the cells incubated in the wells coated with IgG alone. This inhibitory effect of the ESP on IgG-induced superoxide production of granulocytes was concentration-dependent. These results suggest that ESP secreted by PwNEM may be important in the control of effector functions of granulocytes stimulated with IgG in human paragonimiasis.
It has been shown that phagocytes can destroy intra- and extra-cellular parasites in part by the production of toxic oxygen metabolites, such as superoxide and hydrogen peroxide (Bass and Szejda, 1979; Reiner and Kazura, 1982). Involvement of toxic oxygen species in direct killing of the worm by granulocytes has been demonstrated in vitro in some helminth infections, including schistosomula (Kazura et al., 1981) or eggs (Kazura et al., 1985) of Schistosoma mansoni and newborn larvae of Trichinella spiralis (Kazura and Aikawa, 1980). Granulocytes express cell surface receptors for IgG and, therefore, their efficiency in phagocytosis is enhanced when specific IgG is bound to the worms. For example, human granulocytes kill schistosomula of Schistosoma mansoni (Butterworth et al., 1975) and newborn larvae of Trichinella spiralis (Kazura, 1981) in the presence of parasite-specific IgG. Although actual stimuli for the generation of oxygen metabolites of granulocytes in vivo are not completely defined, it has been known that the IgG-coated surface is an effective physiological stimulus for granulocytes (Kaneko et al., 1995). In contrast to effective responses of granulocytes to IgG-coated worms, it is unknown about the role of excretory-secretory product (ESP) released by helminth parasite in effector function of granulocytes stimulated by IgG. The ESP secreted by parasitic worms is presumed to be released in vivo, and is therefore most likely to be involved in direct interaction with host components (Rhoads and Fetterer, 1997). The ESP of Paragonimus westermani newly excysted metacercariae (PwNEM) contains a large quantity of proteolytic enzymes, which play important roles in migration in host tissue (Chung et al., 1995), and immune modulation (Hamajima et al., 1994). In addition, cysteine proteases in the ESP of PwNEM or live metacercarial ESP are capable of degrading human IgG in vitro (Chung et al., 1997). This result led to a speculation that the ESP of PwNEM can attenuate an effector function of granulocytes stimulated with IgG. Therefore, this study was conducted in an attempt to determine the effect of ESP released by PwNEM on superoxide production of granulocytes stimulated with immobilized IgG.
Metacercariae of P. westermani were collected from naturally infected freshwater crayfish, Cambaroides similis, at an endemic area in Korea. Excretory-secretory product (ESP) of P. westermani metacercariae was prepared by transferring 5,000 newly excysted metacercariae into 5 ml physiological saline and incubating at 37℃ in a 5% CO2 incubator for 12 hr. The incubation medium was dialyzed against distilled water and centrifuged at 12,000 rpm for 30 min. The resulting supernatant were lyophilized and diluted with an appropriate medium to the desired concentration immediately before use. The amounts of proteins in the ESP were measured using the bicinchoninic acid protein assay kit (Pierce, IL, USA).
Granulocytes were isolated from the peripheral blood of normal individuals. Granulocytes were isolated by using a gradient percoll solution. Briefly, venous blood anticoagulated with 50 U/ml heparin was diluted with PIPES buffer (25 mM PIPES, 50 mM NaCl, 5 mM KCl, 25 mM NaOH, 5.4 mM glucose, pH 7.4) at a 1:1 ratio. Diluted blood was overlaid on an isotonic Histopaque solution (density, 1.083 g/ml) (Sigma) and centrifuged at 1,000 g for 30 min at 4℃. The supernatant and mononuclear cells at the interface were carefully removed, and erythrocytes in the sediment were lysed by two cycles of hypotonic water lysis. Isolated granulocytes were suspended in the reaction medium. Freshly isolated granulocytes were stimulated by incubating them in a 96-wells plate coated with human IgG (Sigma) in the absence or presence of ESP of PwNEM. Briefly, 96-well tissue culture plates (Nunc, Denmark) were coated with human IgG diluted in PBS at concentrations from 0 to 100 µg/ml in the absence or presence (2, 6, 20 µg) of the ESP for 2 hr at 37℃. After incubation, the wells were aspirated and then washed twice with PBS. Two hundred microliters of cell suspension (2.5 × 105 cell/ml) in HBSS with 10 mM HEPES, 0.03% gelatin and 100 µM cytochrome c (Sigma) were dispensed in the wells coated with IgG in the absence or presence of the ESP. As a positive control, 200 µl of 1 ng/ml PMA in HBSS with 10 mM HEPES were added to the cell suspension dispensed in the wells not coated with IgG in the absence of the ESP. Immediately after the addition of the cells, the absorbance in each well was measured at 550 nm with a micoplate autoreader (Thermomax; Molecular Devices, USA), followed by repeated readings. Between absorbance measurement, the plate was incubated at 37℃. Each reaction was conducted in duplicates and superoxide anion generation was calculated with an extinction coefficient of 21.1 × 10-3 M/cm for reduced cytochrome c at 550 nm, and was expressed as nmoles of cytochrome c reduction/1 × 105 cells. Data are presented as mean±SE from the number of experiments indicated. Statistical significance of the differences between the two groups was assessed with paired Student's t-test.
To investigate whether the ESP of PwNEM could influence the effector function of granulocytes stimulated with IgG, the effect of ESP on superoxide production of granulocyte stimulated with immobilized IgG was tested in vitro. As shown in Fig. 1, granulocytes released superoxide in response to immobolized IgG alone in a time-dependent manner. The kinetics of superoxide production of granulocytes stimulated with three different concentrations (10, 30, 100 µg/ml) of IgG was nearly identical, although the amount of superoxide produced in response to the immobilized IgG was concentration-dependent. Granulocytes, stimulated by 10 µg/ml IgG, produced superoxide which was detectable after 90 min and gradually increased thereafter. Superoxide production of granulocytes stimulated by 30 and 100 µg/ml IgG was detectable after 60 and 45 min, respectively. In contrast, when granulocytes were incubated in non-coated plates, little or no production of superoxide was found. These IgG-induced superoxide production of granulocytes were strongly inhibited when the cells were incubated in the wells coated with IgG in the presence of the ESP (6 or 20 µg) (inhibition range; 73.6-89.6%, p<0.01) (Table 1). However, the inhibitory effect of the lowest amount (2 µg) of the ESP was minimal (IgG 30 µg/ml, 21.3% inhibition; IgG 100 µg/ml, 23% inhibition) (Table 1).
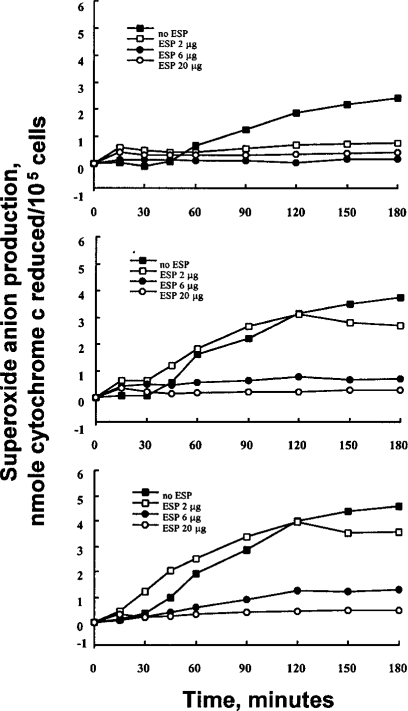
Kinetics of superoxide anion production by granulocytes stimulated with immobilized IgG in the absence or presence of ESP secreted by P. westermani newly excysted metacercariae. Wells of tissue culture plates were coated with various concentrations of IgG (10 µg/ml, top panel; 30 µg/ml, middle panel; 100 µg/ml, bottom panel) with or without ESP. Data are presented as mean values of three independent experiments.

Comparison of the effects of excretory-secretory product (ESP) of P. westermani newly excysted metacercariae on IgG-induced superoxide production by granulocytes
This study shows that the ESP of PwNEM inhibit superoxide production of granulocytes stimulated with immobilized IgG in a dose-dependent manner. In addition, the ESP degraded IgG in vitro in a dose-dependent manner (data not shown). This result suggests that PwNEM secrete some biological molecules that interfere with IgG-stimulated effector functions. Chung et al. (1997) reported that the ESP obtained from 20 newly live excysted metacercariae or purified cysteine proteases from the ESP in vitro cleaved the heavy chain of IgG within 2 hr, and that the secreted metacercarial cysteine proteases of P. westermani revealed stronger activity in cleaving IgG than those of the juveniles and adult worms. Therefore, the inhibitory effect of the ESP on IgG-induced superoxide production of granulocytes is likely due to degradation of IgG induced by cysteine proteolytic activity in the ESP. In addition, it is reported that the cathepsin L proteases secreted by Fasciola hepatica in vitro prevented antibody-mediated eosinophil attachment to newly excysted juvenile (Carmona et al., 1993). Thus, all together, these findings suggest that proteolytic enzyme in the ESP produced by helminth worm is a crucial molecule for evading cell-mediated ADCC against the worms, and that higher amount and activity of proteolytic enzyme in the ESP secreted by younger worms is required for survival of the worms and successful migration to their final destination in parasitic infections.
ACKNOWLEDGEMENT
The author would like to thank Mr. Yong-Moo Won, Department of Parasitology, College of Medicine, Ewha Womans University for his help in collecting freshwater crayfish and isolating P. westermani metacercariae from the crayfish.