Sensitization of Children to Storage Mites in Kutahya, Turkey
Article information
Abstract
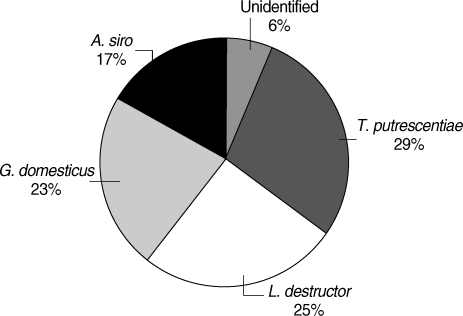
Specific IgE against Acarus siro, Glycphagus domesticus, Tyrophagus putrescentiae, and Lepidoglyphus destructor have been investigated by ELISA in sera of 92 children. Of them, 41 were found to be specific IgE positive (≥ 0.35 IU/ml) against at least one of house dust mite species, Dermatophagoides pteronyssinus and Dermatophagoides farinae, by an immunoblot. In 65.9% of the dust mite-sensitized children, specific IgE against at least one of these mite species was found. Sensitization levels, including co-sensitization cases were found to be 35.7% against A. siro, 24.4% against T. putrescentiae, 31.7% against L. destructor, and 26.8% against G. domesticus. In non-sensitized children, dust mite sensitization level was found to be 25.5%. Breakdown of sensitization by individual species in this group was; against A. siro and T. putrescentiae at 7.8%, against L. destructor at 13.7%, and against G. domesticus at 9.8%. When all children were reckoned, 43.5% was found to be sensitized against at least one storage mite species, with sensitizations against A. siro at 18.5%, T. putrescentiae at 26.1%, L. destructor at 21.7%, and G. domesticus at 17.4%. In dust samples collected from the dwellings of children, distribution of species was found to be A. siro (17%), G. domesticus (23%), T. putrescentiae (29%), L. destructor (25%), and unidentified (6%). In Fisher's chi-square test on SPSS program, there was a relationship between dust mite sensitization and storage mite sensitization (P < 0.05), but no meaningful relationship was found on the basis of individual mite species.
INTRODUCTION
Storage mites (SM) and house dust mites (HDM) are astigmatic organisms and they take almost an inseparable place in modern human life. The article by Voorhost et al. [1] in 1964 that reported mites as the main allergens in dwellings has been an historic milestone in allergy research, and in the aftermath, studies on their biological-ecological properties and allergenic structures have gained momentum [2,3]. Although it is generally accepted that the acceleration in atopic disorders started in the 1960s and 1970s in the industrialized world, which proliferated in the 1980s and 1990s [4]; a study draws attention to reports from the 1920s on sensitization to allergens in the air [5]. Mite feces are the major source of house dust allergens over 95% of the total. These organisms produce about 20 sets of feces in a day [6], and 20% of their body and fecal extract particles are smaller than 5 µm which can easily contact or infiltrate the human body [7].
To date, 19 different allergen types of HDM have been characterized and found to elicit varying degrees of IgE reactivity and T-cell responses [6], and it has been found that allergic reactions against Group I and II antigens developed in 90% of sensitized people. Antigenic structures other than Group I and II may be considered as unstable and minor [8]. It is regarded that more than 20% of world population suffer from diseases which arise from specific IgE [9], and it is pointed out that mites, causing such effects due to their antigenic structures, have varying prevalences in different regions by 20- to 60-folds [10].
In recent years, spread and magnitude of allergic diseases in developed and developing countries have been on the rise which can be explained by an actual 1% rise in the prevalence, especially in children, rather than advancements in diagnosis systems [10]. It has been reported that mites cause slow-progressing diseases like allergic asthma, allergic dermatitis, and allergic rhinitis in atopic people living in industrialized countries in particular [4]. A correlation between exposure of sensitized people to antigen-containing house dust and ensuing allergic symptoms out of specific IgE formation has been stated as very obvious [11].
The storage mites, principally those from Glycyphagus and Acarus genera, may cause some allergic symptoms on farmers, grain dealers, and bakers, and on people who happen to consume contaminated food with them. The effects of these mites may extend to serious allergic reactions, and anaphylactic shocks in some cases [12]. It should be alarming for their significance, since the fecal pellets and body extracts of storage mites are easily transferrable [13] to dwellings, and they can survive and continue their biological cycles under habitable conditions [14].
The present study was carried out to investigate SM sensitization (Acarus siro, Tyrophagus putrescentiae, Lepidoglyphus destructor, and Glycphagus domesticus) in children who had already been searched for D. pteronyssinus and D. farinae sensitization and mite population in their homes; and to obtain a general idea about SM sensitization of children in Kutahya, Turkey.
MATERIALS AND METHODS
Sensitization against house dust mites were investigated in children admitted to Dumlupinar University Hospital Pediatric Clinic, Kutahya, Turkey (Fig. 1) between October 2005 and February 2007 for various health complaints which led to suspicion of allergic rhinitis, allergic dermatitis, and allergic asthma. Children were tested for specific IgE (Rida® Allergy Screen™, R-Biofarm As, Darmstadt, Germany) against Dermatophagoides pteronyssinus and Dermatophagoides farinae in their sera, with 41 found positive out of total 92. Dwellings of these children were scanned for mite presence in house dust.
Sera that had been tested for presence of HDM sensitization were kept for SM sensitization studies in sterilized 1 ml tubes with eppendorf tubes at -86℃ deep freezer (Revco). At a later stage, these samples were defrosted once and centrifuged to be made ready for the new testing. In the tests, specific IgE for A. siro, G. domesticus, T. putrescentiae, and L. destructor were searched in sera of 92 children; 41 of them were found previously to have specific IgE against at least 1 of D. pteronyssinus and D. farinae antigens, while the remaining 51 children were found negative.
Specific antigens of each SM species were taken into antigen disks contained in plates (Art. No. A0079, Lot. No. 01127) at room temperature and 0.1% NaN3 contents were put into pipettes ready for ELISA-testing (Ridascreen®, specific IgE, R-Biofarm AG), in compliance with the company guidelines. Inter-incubation washing works were carried out with an auto-plate washer (Elx-50, Bio-Tek Instruments Inc., Winooski, VT, USA). Following the testing processes, results were obtained by a plate reader (Elx-800, Bio-Tek Instruments Inc.) working at 450 nm. The values with higher than specific IgE concentration of 0.35 IU/ml have been considered sensitized.
House dust specimens had been collected previously, for studying HDM sensitization, with a 1,200 W vacuum cleaner, using a separate dust-bag for each house, at a pace of 2 min per squaremeter. The area vacuumed in these houses included all personal and common household stuffs, such as beds, mattresses, pillows, linens, couches, the flooring, kitchen, and larder, so as to sample the conditions experienced by the subjects thoroughly.
All samples were analyzed by 2 methods. In the first method, samples were analyzed as told by Spieksma and Spieksma-Bozeman [15]. As defined in the reference article, 5 g of sample was boiled with 90% lactic acid in a beaker, and then transferred to glass tubes to be centrifuged for 4 times at 300 g. The resulting supernatants were spread carefully over filter papers (Schleicher & Schuell-Black). In the second method, samples were analyzed as told by Solarz [16]. As defined in the reference article,10 g dust samples were taken into a 600-ml beaker and saturated salty water added over it with a couple of drops of washing detergent. These mixtures were spun in a magnetic mixer for 1 hr and separated from larger particles. The obtained suspensions were left floating for a full day, and dropped slowly over the filter paper, ensuring sediments stay out of the process. The filtrates were washed away with tap water to remove the salt. Some amount of saturated salty water equal to the substance taken from the beaker to the filter paper was added to the beaker again to be floated 3 times for 24 hr.
Filtrates deposited over filter papers in both methods were inspected under a stereomicroscope for mites which were collected by the help of a needle and preserved in a protective solution (15 ml liquid glycerin + 90 ml distilled water + 300 ml 95% ethyl alcohol). To make the preparation, Hoyer solution (50 ml distilled water + 20 ml liquid glycerin + 30 g gum arabicum + 200 g chloral hydrate) was used, and left for dehydration. Mites were identified under a light microscope using the classifications proposed by the authors [3,17,18].
RESULTS
This study found SM sensitization in 65.9% of HDM-sensitized subjects (n = 41) and in 25.5% of non HDM-sensitized subjects (n = 51). In children sensitized against only D. pteronyssinus (n = 3), SM sensitization was found to be at 66.6%, in sensitized children against D. pteronyssinus and D. farinae (n = 38) at 65.8%, and for the whole HDM sensitized group (n = 41) at 67.5% (Table 1). In HDM-sensitized group of children (n = 41), sensitizations were found to be against A. siro at 35.7%, against T. putrescentiae at 24.4%, against L. destructor at 31.7%, and against G. domesticus at 26.8%, including co-sensitizations.
Sensitization level has been found to be at 25.5% in those children (n = 51) who had been found non-sensitized against HDM. In this group of children, sensitizations were found to be against A. siro and T. putrescentiae at 7.8%, against L. destructor at 13.7%, and against G. domesticus at 9.8%. Taking into account of all children (n = 92), sensitizations were found to be against A. siro at 18.5%, against T. putrescentiae at 26.1%, against L. destructor at 21.7%, and against G. domesticus at 17.4%.
In microscopic examinations for SM of the house dust samples taken from the homes of study group, A. siro constituted 17% of the total, G. domesticus 23%, T. putrescentiae 29%, and L. destructor 25%, while 6% of mites could not be identified as their morphological structures were disfigured beyond recognition (Table 2; Fig. 2).
DISCUSSION
Sensitization against A. siro was reported as 2.3-48.12% [19-22], against L. destructor as 3.3-68.24% [19,20,22], against G. domesticus as 3.8-57.14% [21,22], and against T. putrescentiae as 6.2-64.66% [20,22]. In our research, sensitizations were found to be against A. siro at 18.5%, against T. putrescentiae at 26.1%, against L. destructor at 21.7%, and against G. domesticus at 17.4%.
In food production sector employees, SM allergy was found in 14% [23], and in agricultural sector employees in 6.5-56.9% [21,24]. Contrary to comments by Van Hage Hamsten [24] who explained high levels of SM allergy due to subjects' involvement in agricultural sector, there were no agricultural workers among the study group children nor their houses were in agricultural areas.
Van der Heide et al. [25] found a common sensitization against SM in patients sensitized against D. pteronyssinus, and similarly Hunbhayarlah [26] reported strong cross reactions between T. putrescentiae and D. pteronyssinus. Some researchers stated cross reactions of HDM and SM to be limited with those in inhibition tests [23,24,27], while Ventas et al. [28] reported lack of cross reactions with L. destructor and D. pteronyssinus allergens. In our study, although no inhibition test was carried out to determine specific IgE in sera, a statististical assessment of the data suggests no cross reactions (P > 0.05).
Kronoqvist [21] reported a significant rise in asthma and accompanying rhinoconjunctivitis in agricultural workers, but added that the rise in SM sensitization was not high. Marcos et al. [22] pointed that SM sensitization had no correlation to farming, education, gender, humidity, cat or dog presence, and storage side dwelling. Similarly, in our study, too, no correlation between SM sensitization and presence of domestic animals, location of the house, mode of heating, and type of floor covering could be established; moreover, no statistical correlation has been found among SM species and quantities obtained from dwellings of the subjects, and allergic states of them, including both allergic and non-allergic children (P > 0.05).
This study is significant in providing an idea on the level of SM sensitization in children living in Kutahya, Turkey. As a result of the study, SM sensitization was found to be 43.5% among subjects. This study also shows that in HDM-sensitized children have more sensitization against SM compared to non-allergic subjects. In research group children (n = 92), sensitizations were found to be against A. siro at 18.5%, against T. putrescentiae at 26.1%, against L. destructor at 21.7%, and against G. domesticus at 17.4%. Generally, a higher level of SM sensitization was found in children with HDM sensitization; however, no mite species was found to be dominant in SM sensitization (P < 0.05).
Different results obtained in different studies at various institutions may be explained by different occurrences of mites and different sensitizaton levels of individuals. This finding brings forward the need for more attentive consideration of SM besides HDM. Identification of mite species in the study was made microscopically, and no antigen detection methods were employed which might have caused unnoticed mite species in the samples. In future studies, utilization of antigen detection for identification of mite species would be more pertinent.