Cited By
Citations to this article as recorded by

Molecular characterization of nodule worm in a community of Bornean primates
Liesbeth Frias, Danica J. Stark, Milena Salgado Lynn, Senthilvel Nathan, Benoit Goossens, Munehiro Okamoto, Andrew J. J. MacIntosh
Ecology and Evolution.2019; 9(7): 3937.
CrossRef Testing for links between face color and age, dominance status, parity, weight, and intestinal nematode infection in a sample of female Japanese macaques
Lucie Rigaill, Andrew J. J. MacIntosh, James P. Higham, Sandra Winters, Keiko Shimizu, Keiko Mouri, Takafumi Suzumura, Takeshi Furuichi, Cécile Garcia
Prevalence of intestinal parasites with molecular detection and identification of Giardia duodenalis in fecal samples of mammals, birds and zookeepers at Beni-Suef Zoo, Egypt
Asmaa Alaa Kamel, Gihan K. Abdel-Latef
Journal of Parasitic Diseases.2021; 45(3): 695.
CrossRef The Epidemiology of Human Strongyloidiasis
Rubén O. Cimino, Alejandro Krolewiecki
Current Tropical Medicine Reports.2014; 1(4): 216.
CrossRef Human Trichuriasis: Whipworm Genetics, Phylogeny, Transmission and Future Research Directions
Martha Betson, Martin Jensen Søe, Peter Nejsum
Current Tropical Medicine Reports.2015; 2(4): 209.
CrossRef Molecular identification of the strongyloid nematodeOesophagostomum aculeatumin the Asian wild elephantElephas maximus
O. Phuphisut, W. Maipanich, S. Pubampen, M. Yindee, N. Kosoltanapiwat, S. Nuamtanong, A. Ponlawat, P. Adisakwattana
Journal of Helminthology.2016; 90(4): 434.
CrossRef Trichuris infection in captive non-human primates in zoological gardens in Spain
J. Rivero, R. Callejón, A. M. García-Sánchez
Journal of Helminthology.2025;[Epub]
CrossRef Novel insight into the genetic diversity of strongylid nematodes infecting South-East and East Asian primates
Bethan Mason, Barbora Cervena, Liesbeth Frias, Benoit Goossens, Hideo Hasegawa, Kenneth Keuk, Abdullah Langgeng, Kasia Majewski, Takashi Matsumoto, Keiko Matsuura, Renata Mendonça, Munehiro Okamoto, Steve Peter, Klara J. Petrzelkova, Symphorosa Sipangkui,
Parasitology.2024; 151(5): 514.
CrossRef Genetic characterization of nodular worm infections in Asian Apes
Erhan Yalcindag, Peter Stuart, Hideo Hasegawa, Adrian Streit, Jana Doležalová, Helen Morrogh-Bernard, Susan M. Cheyne, Wisnu Nurcahyo, Ivona Foitová
Emerging zoonotic diseases originating in mammals: a systematic review of effects of anthropogenic land‐use change
Rebekah J. White, Orly Razgour
Mammal Review.2020; 50(4): 336.
CrossRef Fecal metagenomics for the simultaneous assessment of diet, parasites, and population genetics of an understudied primate
Amrita Srivathsan, Andie Ang, Alfried P. Vogler, Rudolf Meier
Frontiers in Zoology.2016;[Epub]
CrossRef Pathologic characteristics of infectious diseases in macaque monkeys used in biomedical and toxicologic studies
Etsuko Ohta
Journal of Toxicologic Pathology.2023; 36(2): 95.
CrossRef Monkeys in the Middle: Parasite Transmission through the Social Network of a Wild Primate
Andrew J. J. MacIntosh, Armand Jacobs, Cécile Garcia, Keiko Shimizu, Keiko Mouri, Michael A. Huffman, Alexander D. Hernandez, Judith Korb
Molecular identification of Trichuris trichiura and Hymenolepis diminuta in long-tailed macaques (Macaca fascicularis) in Lopburi, Thailand
Wanat Sricharern, Tawin Inpankaew, Sarawan Kaewmongkol, Thitichai Jarudecha, Natnaree Inthong
Veterinary World.2021; 14(4): 884.
CrossRef Trichuris trichiura Incidentally Detected by Colonoscopy and Identified by a Genetic Analysis
Yuto Ishizaki, Kazumasa Kawashima, Naohiko Gunji, Michio Onizawa, Takuto Hikichi, Mitsuko Hasegawa, Hiromasa Ohira
Internal Medicine.2022; 61(6): 821.
CrossRef Ecology and Epidemiology of Nematode Infection in Japanese Macaques:
Andrew James Jonathan MacIntosh
Primate Research.2014; 30(1): 23.
CrossRef Trichuris trichiura (Linnaeus, 1771) From Human and Non-human Primates: Morphology, Biometry, Host Specificity, Molecular Characterization, and Phylogeny
Julia Rivero, Cristina Cutillas, Rocío Callejón
Frontiers in Veterinary Science.2021;[Epub]
CrossRef Nuclear and Mitochondrial Data on Trichuris from Macaca fuscata Support Evidence of Host Specificity
Serena Cavallero, Margherita Montalbano Di Filippo, Silvia Rondón, Claudio De Liberato, Stefano D’Amelio, Klaus G. Friedrich, Federica Berrilli
A Review of Strongyloides spp. Environmental Sources Worldwide
Mae A. F. White, Harriet Whiley, Kirstin E. Ross
Trichuris spp. in Animals, with Specific Reference to Neo-Tropical Rodents
Kegan Romelle Jones
Veterinary Sciences.2021; 8(2): 15.
CrossRef Microspheres as Surrogate Helminth Eggs: A Comparative Labscale Sedimentation Study for Tap- and Wastewater
Barbara K. Arthur, Edith Nettmann, Andrea Rademacher, Manfred Lübken, Bernd Marschner, Marc Wichern
Abstract
Natural habitat fragmentation and reducing habitat quality have resulted in an increased appearance of Japanese macaques, Macaca fuscata (Gray, 1870), in suburban areas in Japan. To investigate the risk of zoonotic infections, a coprological survey of helminth eggs passed by wild Japanese macaques was carried out in 2009 and 2010 in Shiga Prefecture, Japan. Microscopic examination found helminth eggs in high prevalence, and nucleotide sequencing of DNA extracted from the eggs identified Oesophagostomum cf. aculeatum and Trichuris trichiura. A fecal culture also detected infective larvae of Strongyloides fuelleborni. These zoonotic nematodes pose a potential health issue to local people in areas frequented by Japanese macaques.
Key words: Oesophagostomum aculeatum, Trichuris trichiura, Strongyloides fuelleborni, Japanese macaque, zoonosis
The Japanese macaque,
Macaca fuscata (Gray, 1870), is unique to the Japanese archipelago and is the only non-human primate that inhabits these islands, with an estimated total population of 100,000 [
1]. Advancing forest habitat fragmentation and reducing habitat quality in recent years have forced Japanese macaques to appear frequently at forest edges and in farmland near residential areas. Japanese macaques carry some helminths potentially zoonotic to humans, including nematodes, such as
Oesophagostomum aculeatum,
Strongyloides fuelleborni, and
Trichuris trichiura [
1,
2]. Traditionally, however, specific diagnosis of gastrointestinal nematode infections in wild non-human primates is based on the detection of eggs in feces, where the morphological characteristics of ova alone may not be reliable enough for species identification. Thus, to identify the helminths carried by wild Japanese macaques, and to evaluate the risk of zoonotic infections to humans, we carried out molecular identification of eggs passed in feces by sequencing some nuclear targets.
Fecal samples were collected in May 2009 and June 2010 from the ground at the foot of mountains in Takashima city, Shiga Prefecture, Japan. This district is a rather sparsely-populated area adjacent to farmland that is frequented by free-roaming macaques at irregular intervals. That the fecal samples were derived from Japanese macaques was confirmed by local residents who had often seen monkeys and their feces at very close proximity to their houses. A total of 36 fecal samples were collected in 2009 and 2010. Although the length of time the feces had remained on the ground could not be determined, they appeared to have been there only for a few days.
The fecal samples, 2 g each, were dissolved in 200 ml of distilled water, sieved through 2 sheets of cotton gauze, and left to stand for 1-2 hr to obtain precipitates. After 2 additional cycles of dissolution and sieving, the precipitates finally obtained were subjected to microscopic examination for helminth eggs. One fecal sample was subjected to fecal culture using a filter paper test-tube method.
To molecularly identify helminth eggs, 10-30 helminth eggs (
Oesophagostomum sp. or
Trichuris sp.) were collected under a stereoscopic microscope. Approximately 50
Strongyloides filariform larvae were collected from the bottom of culture test tubes. DNA was extracted using TaKaRa DEXPAT® (Takara Bio Inc., Shiga, Japan), which was originally designed to retrieve small amounts of DNA from paraffin embedded tissue sections. The primers used for PCR amplification were: NC1 and NC2 [
3] for internal transcribed spacer (ITS)2 of
Oesophagostomum spp.; 5'-CAAGGTTTTCGTAGGTGAA-3' and 5'-CTCTTCATCGACCTATGAAC-3' for ITS1 of
Strongyloides spp.; and 5'-CTAAGCAGAGCCTTAAATT-3' and 5'-TCCGCTTAACGATATGCTTA-3' for ITS2 of
Strongyloides spp. The 18S rRNA of
Trichuris spp. was amplified in 4 overlapping fragments using the forward and reverse primers 5'-AAGCCGCGAATGGCTCATTA-3' and 5'-CTGCTGCCTTCCTTGGATGT-3' for the first fragment, 5'-CCATGGTGACAACGGTTAAC-3' and 5'-ATTGGTCGTCTTGCTGCGAT-3' for the second fragment, 5'-ACGGGGACATTCGTATTGCT-3' and 5'-GCTAGTTAGTAGGCCAGAGT-3' for the third fragment, and 5'-TTCAGTGGGTAGTGGTGCAT-3' and 5'-CCTACGGAAACCTTGTTACG-3' for the last fragment. The amplified products were directly sequenced on both strands. The nucleotide sequences determined in this study were deposited in DNA databases with the accession numbers indicated in the text, Table, and/or Figures.
Microscopic examination revealed eggs similar to those of hookworms and eggs of Trichuris sp. in 50% and 38% of 18 fecal samples examined in 2009, and 38% and 31% of 18 fecal samples examined in 2010. Eggs similar to those of Strongyloides sp. were also found in a few fecal samples in both years. Fecal cultures of 1 specimen collected in 2010 produced filariform larvae of Strongyloides sp. Cysts of Entamoeba coli were also occasionally found.
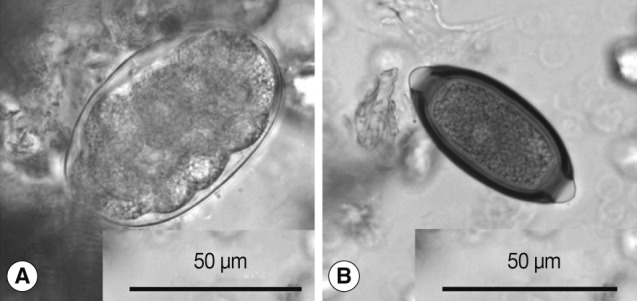
Hookworm-like eggs found in the present survey were thin-shelled, ovoidal, segmented into ≥8 cells, and measured 69-78 by 41-48 (mean: 74×45) µm (
Fig. 1A). Non-human primates harbor strongylid nematodes, such as
Oesophagostomum spp. and
Ternidens spp., the eggs of which are hardly distinguishable from those of hookworms. Within these species,
O. aculeatum is confined to Asia and infects primates, such as Japanese macaques and cynomolgus monkey (
Macaca fascicularis) [
4-
7], while
Oesophagostomum bifurcum and
Oesophagostomum stephanostomum infect African primates as well as rhesus monkeys (
Macaca mulatta) in Asia [
3,
6,
8,
9].
Ternidens deminutus occurs in primates in Asia and Africa [
5,
10,
11], but has not been recorded in Japanese macaques. Thus, the type of ova found in the present study has been traditionally identified as that of
O. aculeatum, as long as it was derived from Japanese macaques [
1,
2]. Indeed, the dimensions of the hookworm-like eggs found in the present study were compatible with those of
O. aculeatum (69-86 by 35-55 µm), the measurements of which were based on intrauterine eggs from
O. aculeatum female worms, but were larger than those of
T. deminutus (57-65 by 36-45 µm) [
4].
ITS2 sequencing was carried out in 2 specimens containing hookworm-like eggs collected in 2009 and 2010. Sequences obtained in both specimens were identical and deposited in public databases (AB586134). Unfortunately, we could neither obtain morphologically identified
O. aculeatum adult worms for sequencing, nor find any reference sequence of
O. aculeatum in the literature or DNA databases. Nevertheless, a BLAST search against the EMBL databases identified sequences of
Oesophagostomum sp. from
M. fuscata (HM067976) with 99.0% identity, followed by those of
O. bifurcum and other
Oesophagostomum species with identities≤94.0%. Pairwise comparisons of ITS2 sequences showed that the putative
O. aculeatum from Japanese macaques were clearly distinguished from
O. bifurcum and
O. stephanostomum with genetic distances equal to or over 0.047, and from
T. deminutus with genetic distances of ≥0.056 (
Table 1). Thus, we preliminarily identified the eggs as
Oesophagostomum cf.
aculeatum until it could be verified by sequencing nucleotides of morphologically identified
O. aculeatum adult worms.
Trichuris eggs obtained in the present study measured 58-63×28-31 (mean: 61×29) µm (
Fig. 1B). The eggs of human
T. trichiura are divided into 2 morphotypes; the common type with a dimension of 56±2 by 26±1 µm, and the less common type with larger dimensions (75±4 by 31±2 µm) [
12]. The eggs of Japanese macaque-origin were similar to those of the common variety of
T. trichiura eggs. Sequencing of 18S rRNA in
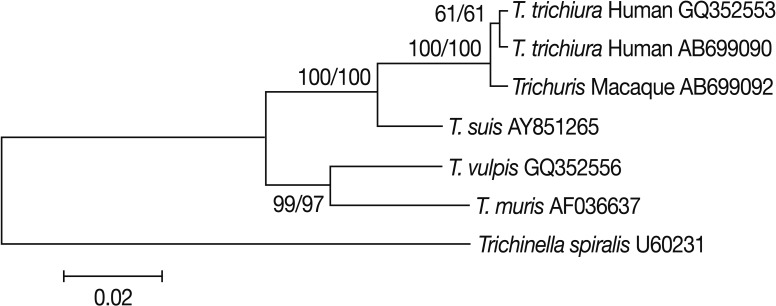
Trichuris eggs from Japanese macaques and in 2 adult worms of
T. trichiura from 2 Japanese patients as a reference was carried out. Pairwise comparisons showed that
Trichuris derived from Japanese macaques (AB699092) differed from
T. trichiura from 2 Japanese patients (AB699090) and another
T. trichiura worm of human origin (GQ352553) with genetic distances of 0.003 and 0.005, respectively. Its genetic distances from
Trichuris suis,
Trichuris vulpis, and
Trichuris muris were ≥0.028. Phylogenetic analyses showed that
Trichuris eggs from Japanese macaques clustered unequivocally with
T. trichiura of human origin (
Fig. 2). From these results, we identified the eggs as
T. trichiura.
Filariform larvae of Strongyloides sp. produced in a fecal culture of monkey feces were 502-603 µm in length with a body width of 13-16 µm. The esophagus accounted for 40-45% of body length. The tail measured 66-82 µm in length. ITS1 sequences of the filariform larvae obtained in the present study (AB699093) were similar to those of S. fuelleborni (AB272235, U43581), with genetic distances of 0.013-0.019, while they showed larger differences from Strongyloides callosciureus (AB272229), Strongyloides procyonis (AB205054), Strongyloides stercoralis (U43578), Strongyloides ratti (U43580), and Strongyloides cebus (AB272236), with genetic distances of ≥0.165. ITS2 sequences of the present isolates (AB699094) were also similar to those of S. fuelleborni (AB272235) with a genetic distance of 0.009. Thus, the filariform larvae in the present study were identified as S. fuelleborni.
The present coprological survey of wild Japanese macaques detected eggs of
Oesophagostomum. cf.
aculeatum and
T. trichiura at a rather high prevalence, as well as infective larvae of
S. fuelleborni, confirming previous reports that the eggs of
O. aculeatum,
T. trichiura, and
S. fuelleborni were found in 30.4%, 60.1%, and 29.0% of Japanese macaques, respectively [
1].
Oesophagostomum infections in humans result in single or multiple nodular lesions mainly in the colon and sometimes in extraintestinal tissues. A number of
O. bifurcum infections in humans have been reported, with the majority from Africa, especially northern Togo and Ghana [
9,
13]. A few cases of possible
O. aculeatum infections have also been reported in Southeast Asia [
14-
16]. Except for those cases in Togo and Ghana, larvae often do not complete development in the human body and juveniles remain in their intestinal nodules, which makes diagnosis difficult. The infection of humans with
Oesophagostomum spp. is considered to occur via ingestion of the 3rd-stage larvae in contaminated water, food, and soil. Although no
Oesophagostomum infection in humans has been reported in Japan, people should be aware of the risk of accidental infection.
It has been widely accepted that the
Trichuris species, which infects non-human primates, is similar to that in man and cross infection to humans is possible [
6]. Indeed, previous studies showed that experimental infection of man with
Trichuris ova from Japanese macaques resulted in patent infection [
17]. The present phylogenetic analyses based on 18S rRNA sequences showed a very close relationship of
Trichuris of macaque-origin to that of human-origin, with minor genetic divergence. Previous studies by scanning electron microscopy showed that
T. trichiura from non-human primates exhibited some differences in pericloacal papillae from those of human origin; although the authors considered the findings insufficient to create a new species [
18]. Thus, it remains to be elucidated whether population genetic substructuring exists within
T. trichiura from different primate hosts by analyzing such as mitochondrial DNA sequences, and more studies are needed to clarify whether
T. trichiura of macaque-origin accounts for at least some cases of human trichuriasis.
S. fuelleborni is zoonotic: it has been reported that 3 out of 10 cases of human strongyloidiasis in Zambia were due to
S. fuelleborni [
19-
21]. However, genetic substructuring of
S. fuelleborni exists worldwide according to geographical localities [
21], and further studies are needed to clarify whether infectivity of
S. fuelleborni of Japanese-macaque origin to humans is similar to those from different localities.
Besides those helminth ova found in the present study, eggs of the tapeworm
Bertiella studeri have been detected in 8.9% of fecal samples from Japanese macaques [
1], and at least 5 cases of accidental infection of humans with
B. studeri have occurred in Japan [
22,
23]. Non-human primates are also potential reservoirs for zoonotic transmission of enteric protozoans. Indeed,
Giardia intestinalis (assemblage B) and
Entamoeba spp., such as
E. dispar and
E. nuttalli, have been isolated from Japanese macaques [
24-
26].
In conclusion, the present coprological survey of wild Japanese macaques identified Oesophagostomum cf. aculeatum, T. trichiura, and S. fuelleborni based on sequencing nuclear targets. Further accumulation of sequence data will lead to the identification of these parasites from Japanese macaques based upon more solid evidence. Although further studies are needed to determine the actual risk of zoonotic transmissions of helminths between Japanese macaques and humans, people and health workers should be aware of the potential risk of accidental zoonotic helminth infections in humans living in areas frequented by Japanese macaques.