INTRODUCTION
Toxoplasma gondii is an ubiquitous obligate intracellular parasite, which infects both humans and warm-blooded animals as a zoonotic pathogen widespread in nature [1,2]. Approximately one-third of humans worldwide are estimated to be chronically infected with T. gondii [2,3]. However, the prevalence of the disease and the sources of infection vary among geographic regions with different toxoplasmic environments, i.e., diffenrent climates, eating habits, and hygiene status [4-7].
In high T. gondii endemic regions of the U.S. and European nations, toxoplasmic retinochoroiditis is the major cause of visual impairment which accounts for 30-55% of posterior uveitis [8,9]. For many years, ocular toxoplasmosis was considered to be the result of recurrence of the congenital form of the disease [10]. However, more recent reports support the view that acquired infections might be a more important cause of ocular diseases than congenital ones [11-13]. Clinical features are quite different from each other in that the congenital form tends to show bilateral macular lesions, which is quite different from typical ocular features which is focal retinitis adjacent to a chorioretinal scar or even without the presence of a scar.
Ocular toxoplasmosis is a progressive and recurring necrotizing retinitis, with vision-threatening complications such as retinal detachment, choroidal neovascularization, and glaucoma, which may occur at any time during the clinical course. There lies a matter of controversy about diagnosis and treatment for ocular toxoplasmosis, and to date, many treatment options are applied clinically. This review briefly discusses on clinical features and currently available chemotherapy of ocular toxoplasmosis.
ROUTES OF INFECTION WITH TOXOPLASMA GONDII
T. gondii exists in 3 forms, all of which are possible to infect hosts as a form of zoonosis. Tachyzoites can infect almost all nucleated cells through a process of active invasion, tissue cysts (containing bradyzoites) are formed primarily in the brain and skeletal muscles during the chronic phase of infection, and oocysts are produced during the sexual cycle that takes place in the intestine of acutely infected felines [2,3]. The main routes of infection have been thought to be by ingestion of oocytes from the cat feces present in soil and sand boxes. Oocysts attached to fruits and vegetables and oocysts in water which might be resulted from the process of washing may be a route of infection. However, ingestion of tissue cysts in raw or undercooked meat from several intermediate animals may be the main cause of infection in some countries [7]. A recent study in Korea reported that 5 of 10 active ocular toxoplasmosis patients had a definite history of consuming wild boar meat or deer blood [14]. In addition, evidences show that contaminated drinking water could be a principal route of endemic infections with T. gondii [15,16].
Ocular toxoplasmosis is thought to be due to either congenital or acquired infection. During the congenital infection, the fetus is infected via placental bloodstream, whereas during the acquired infection, parasite transfer is mediated typically through the gastrointestinal tract. Postnatal infection is now thought to be a more common cause of ocular toxoplasmosis [2,11,17].
PATHOGENESIS AND PATHOLOGY OF OCULAR TOXOPLASMOSIS
Most of acute systemic toxoplasmosis in normal hosts tend to be subclinical, but some may present with mild flu-like symptoms. If parasites reach an eye and they yield a focus of inflammation, the lesion is progressed to retinitis and involves the choroid secondarily. Immune responses of the host appear to induce conversion of the parasitic forms, from tachyzoites to bradyzoites and their encystment [18]. The cyst may remain inactive in the scar or nearby for a long time. However, when the cyst ruptures with release of organisms into the surrounding retina, retinitis may be reactivated [19]. The reactivation of retinitis is known to develop at the border of old scars and is attributed to the rupture of tissue cysts which are located within old lesions. Sometimes, however, new lesions are found at locations distant from old scars.
Exact mechanisms of these findings in ocular toxoplasmic patients are yet unknown. Recently, Silveira et al. [20] reported that T. gondii was found in the peripheral blood of acutely and chronically infected patients regardless of the presence of toxoplasmic retinochoroiditis. This indicates that the parasite may circulate in the blood of immunocompetent individuals and the parasitemia could be associated with reactivation of the ocular disease [20,21].
Some studies have suggested a possible route of infection from the brain to the eye through the optic nerve; however, now ocular infection is most likely mediated via the bloodstream [22]. Norose et al. [22] described that the kinetics of parasite load in various areas of the eye revealed that parasite detection in the retina and choroid precedes detection of parasites in the optic nerve, arguing against the optic nerve theory as the main port of entry into the eye. In addition, a hematogenous route of dissemination into the eye is supported by the fact that ocular toxoplasmosis can occur in the absence of toxoplasmic encephalitis [2]. Smith et al. [23] have found that retinal vascular endothelial cells are more readily infected with T. gondii compared with endothelial cells from the other sites of the body, which suggests a preferential infection of the retina by the parasite.
In common, the hallmark of the ocular lesion is retinitis, adjacent to an inactive retinochoroidal scar. The necrosis of the retina and choroid with destruction of the surrounding tissues is found within the active lesion. The inflammatory response is mononuclear cell reaction in nature, and consists of lymphocytes and macrophages at the edge of the lesion. Viable and intact cysts may be present, either adjacent to the scars or within the area of retinal necrosis, and rarely tachyzoites may be identified in the extracelluar space [24].
CLINICAL FEATURES OF OCULAR TOXOPLASMOSIS
The seropositivity for T. gondii infection is relatively high worldwide and the presence of antibodies to T. gondii is useful only to confirm previous exposures to the parasite. This seropositive finding, however, cannot confirm the diagnosis of ocular toxoplasmosis. Therefore, the best clue to diagnosis is recognition of a variety of clinical presentations [7].
Ocular toxoplasmosis most often presents as a focal necrotizing retinitis. It is generally associated with vitritis and often with anterior uveitis. Less commonly, it may present as a papillitis. The age of the first attack of ocular toxoplasmosis is typically in the second decade and during a long-term follow-up, 5-year recurrence rate was 79%, and some patients had multiple recurrences [25,26]. The severity of anterior uveitis can range from a quiet anterior chamber reaction to an intense anterior uveitis, masking the inflammation of the posterior segment. It can be either granulomatous or non-granulomatous inflammations. Also, intense anterior inflammation may occur secondary to retinochoroiditis, near the ora serrata, which may be missed at initial examinations [27,28].
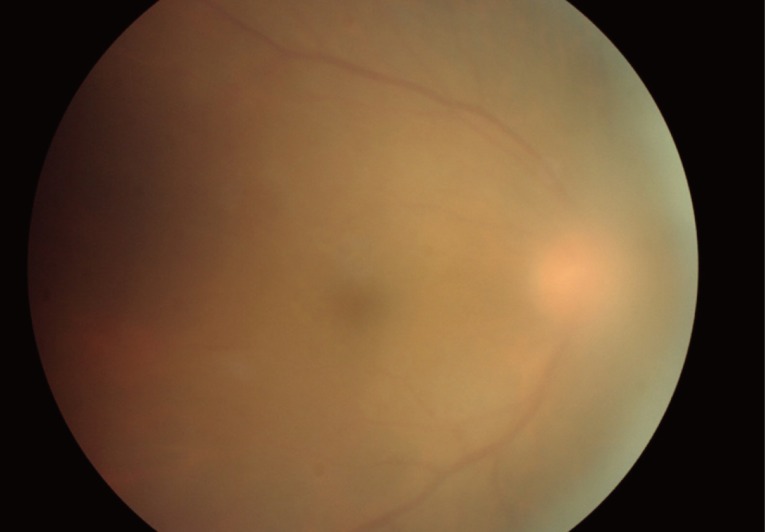
In children with congenital toxoplasmosis, cataracts may occur as a complication of retinochoroiditis, and may follow severe iridocyclitis. Cataract may cause severe amblyopia in children and may need to be removed surgically [29]. Inflammation of the vitreous is usually more intense near the lesion of active retinochoroiditis. In cases of intense vitritis, epiretinal membranes may develop and vitreoretinal traction adjacent to the area may occur. A bright white reflex seen when one shines the light of the indirect ophthalmoscope into the back of the eye is called "headlight in the fog" which results from severe vitritis (Fig. 1).
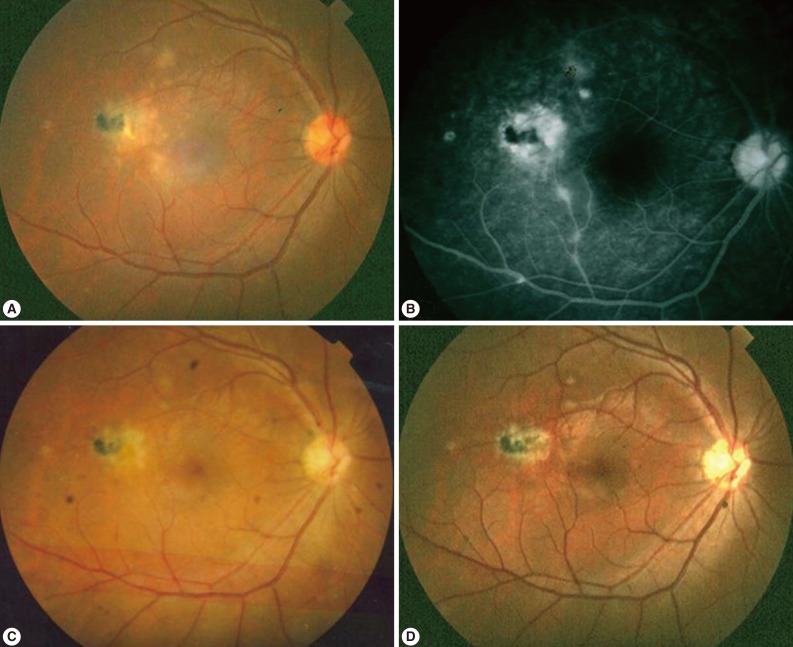
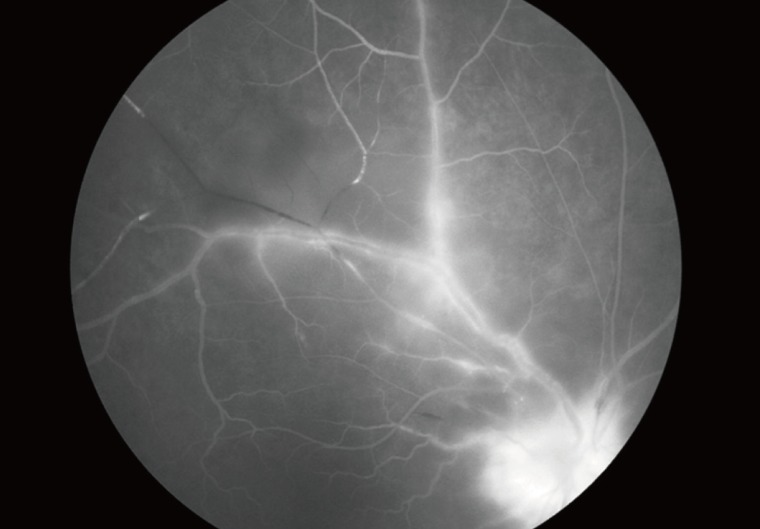
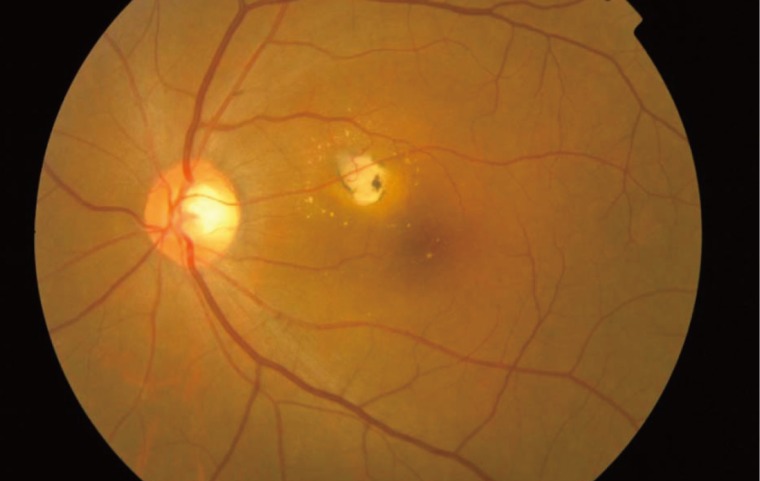
In typical cases, active lesions are seen as whitish foci of retinochoroiditis, frequently adjacent to a pigmented and/or atrophic scar (Fig. 2A-C). An active retinochoroidal lesion usually results in an atrophic retinochoroidal scar, which resolves from the periphery to the center of the lesion (Fig. 2D). Minority of patients with ocular toxoplasmosis may develop fociof inflammation within or directly adjacent to the optic nerve head. Whitish inflammatory lesions located on the disc with associated vitritis suggest the diagnosis (Fig. 3).
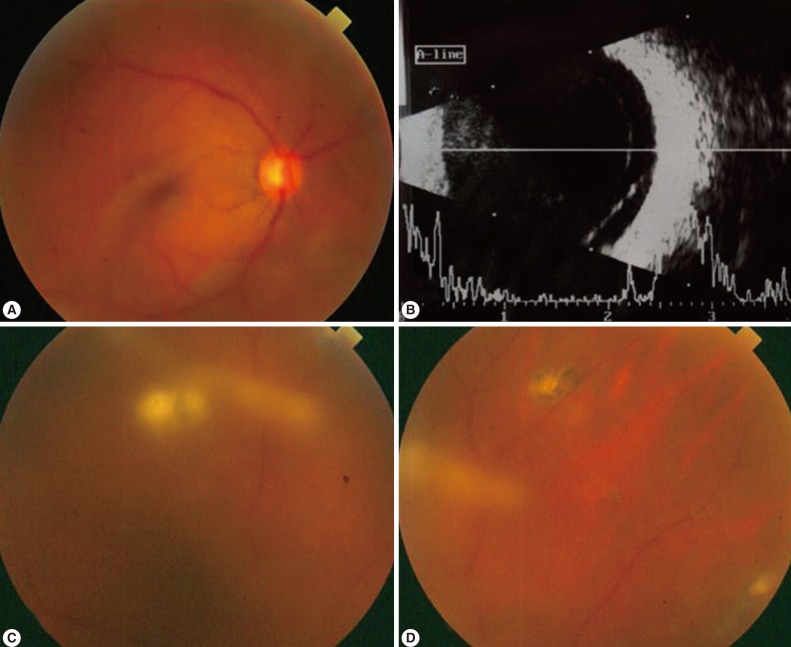
The complications of ocular toxoplasmosis include chronic iridocyclitis, cataract formation, secondary glaucoma, band keratopathy, cystoid macular edema, retinal detachment (Fig. 4), and optic atrophy secondary to optic nerve involvement. Choroidal neovascularization has been described as a late complication of ocular toxoplasmosis (Fig. 5). Other retinal vascular lesions, described as complicating toxoplasmosis, include branch artery occlusion, periphlebitis, and toxoplasmic scleritis.
DIAGNOSIS AND TREATMENT OF OCULAR TOXOPLASMOSIS
The diagnosis of ocular toxoplasmosis is made by ophthalmic examinations and a variety of clinical presentations that are consistent with T. gondii infection of the retina. When this clinical diagnosis cannot be made definitely by a fundoscopic examination, detection of increased T. gondii antibody titers in ocular fluids or amplication of T. gondii DNA have been used successfully to confirm the diagnosis [30,31].
As ocular toxoplasmosis is a self-limiting disease, some clinicians will not treat small peripheral lesions. The aim of the treatment is to arrest parasite multiplication during the active period of retinochoroiditis and to minimize damage to the retina and optic disc [32,33]. To date, there are many treatment options, but the classical chemotherapy using pyrimethamine and sulfadiazine with corticosteroids continues to be the most widely used method [34].
An evidence-based systematic review investigated the effectiveness of systematic antibiotic treatment. Two Cochrane reviews have been established for ocular toxoplasmosis [35,36]. The first review examined whether antibiotics are effective in the treatment of toxoplasmic retinochoroiditis [35]. A recent further Cochrane review assessed the effect of steroid administeration locally or systemically, with or without use of antibiotics [36]. The Cochrane reviews were justified because there is a controversy regarding the use of antibiotics in the peripheral lesions. Also, the course of ocular toxoplasmosis in patients without treatment is variable; some patients can recover in a few weeks without antibiotics. However, there is an argument to use antibiotics because antibiotics can reduce the number of recurrences and antibiotics could also help to speed resolution of inflammation [37]. In this regard, most uveitis specialists agree that antibiotic treatment of toxoplasmic retinochoroiditis is reasonable, although there is no consensus regarding the best treatment regimens [32].
The most frequent chemotherapeutic regimen for ocular toxoplasmosis consists of pyrimethamine and sulfadiazine, plus corticosteroids. This classical treatment may have some risks that depend on patient susceptibility to drug toxicity or allergic reactions. There was a trial to make a combination with less adverse effects and good compliance (due to the reduced number of daily pills). Trimethoprim/sulfamethoxazole plus oral prednisolone is an alternative treatment option. This treatment was shown recently to have similar efficacy to classical therapy in a randomized clinical trial [38,39]. Other treatment option is intravitreal clindamycin injection and dexamethasone which is a promising approach [39-41].
Intravitreal drug administration bypasses ocular barriers, and thereby delivering a high drug concentration directly to the intraocular tissues, avoiding systemic exposure and its risk of complications. Therefore, intravitreal treatment could be more convenient, having a better safety profile and producing a greater drug availability, and result in fewer follow-up visits and hematologic evaluations [39,40]. Soheilian et al. [40] reported that the mean number of injections was 1.6, given every 2 weeks; others suggested weekly injections. Having a good intracellular penetration, clindamycin penetrates cells well and provides a high intracellular/extracelluar ratio compared to other antibiotics, such as erythromycin and levofloxacin [42]. Clindamycin 1.5 mg, given intravitreally, was non-toxic to the retina and had a half-life of 5.6 days. Following 1 mg intravitreal clindamycin injection, its concentration remained≥1.6 µg/ml during about 40 hr, which was higher than the 50% inhibitory concentration for T. gondii [39,42].
Surgery for severe complications of ocular toxoplasmosis has been tried. For this, it is important to lower the burdens of T. gondii by sufficient administration of antibiotics because surgical stress might aggravate clinical symptoms and increase the recurrence rate of ocular toxoplamosis. In addition, we could sample the ocular fluid directly during ocular surgery to confirm the diagnosis of ocular toxoplasmosis.
CONCLUSIONS
Although in some countries, including Korea and Japan, ocular toxoplasmosis is not a common cause of infectious posterior uveitis, ocular toxoplasmosis is one of the most common types (30-50%) of infectious uveitis, affecting the posterior pole in those countries of high endemicity of T. gondii infection, among 30-80% of human population [9,14,43,44].
Ocular toxoplasmosis is an ocular disease that is mainly acquired postnatally. Although food is considered as the source of infection with parasites, contaminated water must also be considered as a mechanism of acquisition of the disease. Proper diagnosis relies on typical clinical findings, especially local retinochoroiditis with or without pre-existing retinochoroidal scar and overlying vitritis.
There are still many therapeutic options for ocular toxoplasmosis, but clinical trials suggest new treatment options that involve lower doses of drugs, facilitating patient compliance and reducing costs and adverse effects. Intravitreal therapy may be promising for treatment of ocular toxoplasmosis. A better clinical and pathophysiological understanding can lead to more effective strategies to prevent and treat the common cause of vision loss. In addition, during the surgical treatment of complications, sufficient administration of antibiotics before surgery is important.