Cited By
Citations to this article as recorded by

Unlocking the Biological Enigma: Influence of Host Length and Infection Site on Parasite Abundance in Ompok bimaculatus
Jit Marick, Subha Shankar Mukherjee, Bhairab Kumar Patra, Anirban Ash
Acta Parasitologica.2024; 69(3): 1492.
CrossRef Morphological and molecular analysis of Isoparorchis trisimilitubis from catfish in Northeast India on the basis of nuclear and mitochondrial DNA
Damanbha Lyngdoh, Calvin D. Warjri, Philayung ZAS, George C. Shabong
Journal of Parasitic Diseases.2023; 47(3): 671.
CrossRef Epidemiological studies of Isoparorchis hypselobagri (Digenea: Trematoda) infecting freshwater catfish Wallago attu in the Aligarh region of Uttar Pradesh
Anam Sahreen, Mohammad Khalid Saifullah
Journal of Parasitic Diseases.2024; 48(3): 642.
CrossRef Infestation with metacercarial stage of Isoparorchis hypselobagri (Billet, 1898) in cage cultured Ompok bimaculatus vis-a-vis host and environmental interaction in a large tropical reservoir
Manoharmayum Shaya Devi, Gunjan Karnatak, Basanta Kumar Das, Asit Kumar Bera, Nilemesh Das, Chayna Jana, Mishal Puthiyottil, Tasso Tayung, Bijay Kumar Behera, Uttam Kumar Sarkar, Yusuf Ali
Parasites of zoonotic interest in selected edible freshwater fish imported to Australia
Michelle Williams, Marta Hernandez-Jover, Shokoofeh Shamsi
Food and Waterborne Parasitology.2022; 26: e00138.
CrossRef A new second intermediate host and phylogenetic relationships based on the ITS2 sequence of Isoparorchis sp. (Digenea: Isoparorchiidae) in Thailand
P. Jaruboonyakorn, T. Chontananarth
Journal of Helminthology.2021;[Epub]
CrossRef Spawning Season and Growth of Korean Dark Sleeper, Odontobutis platycephala in Jaho Stream, Korea1
Hwa-Keun Byeon
Korean Journal of Environment and Ecology.2024; 38(2): 148.
CrossRef Feeding Habits of Korean Dark Sleeper, Odontobutis platycephala in the Jaho Stream, Korea1
Hwa-Keun Byeon
Korean Journal of Environment and Ecology.2024; 38(4): 367.
CrossRef Parasites in Imported Edible Fish and a Systematic Review of the Pathophysiology of Infection and the Potential Threat to Australian Native Aquatic Species
Michelle Williams, Marta Hernandez-Jover, Shokoofeh Shamsi
Abstract
We described here the morphological characteristics for the species identification and fish hosts of Isoparorchis sp. (Digenea: Isoparorchiidae) in the Republic of Korea (Korea). Total 1,371 freshwater fishes collected in Yangcheon (Stream) in Sancheong-gun, Gyeongsangnam-do were examined by the artificial digestion methods to survey the infection status of digenetic trematode metacercariae for 4 years (2013–2016). Adult and larval worms of Isoparorchis sp. were detected in 38 (8.4%) out of 451 fish in 4 species, i.e., Pungtungia herzi, Acheilognathus koreensis, Squalidus japonicus coreanus and Odontobutis platycephala, examined. The infection density was 1.1 worm per fish infected. They were mainly found in the subcutaneous tissues and abdominal cavities. Nodules with worms in the subcutaneous tissues were revealed as the blue ink-colored bulges. Adults leaf-like, 21.6×9.84 mm in average size. The ratio of body length to body width was 2.20: 1. Oral sucker subterminal, 1.03×1.22 mm. Pharynx muscular, 0.55×0.54 mm. Esophagus very short. Ceca convoluted, terminated near the posterior end. Ventral sucker anterior 1/3.75, 1.99×2.10 mm. The ratio of ventral sucker to oral sucker was 1.74: 1. Testes round to elliptical, both sides of ventral sucker, 1.43×1.33 mm. Vitellaria highly dendritic, posterior 1/3 level. Eggs operculated, embryonated, 52×32 μm in size. By the present study, 4 fish species aforementioned are to be listed as the fish hosts of Isoparorchis sp. in Korea and additionally the morphological characteristics are to be described for the species identification.
Key words: Isoparorchis sp., Pungtungia herzi, Acheilognathus koreensis, Squalidus japonicus coreanus, Odontobutis platycephala
Isoparorchis sp. (Digenea: Isoparorchiidae) is a fish fluke, which is associated with the ink spot disease in variety of fish hosts [
1,
2]. This fluke species has been reported in several Asian countries, i.e., Japan, China, the Republic of Korea (Korea), Vietnam, Indonesia, Bangladesh, Pakistan and India, and Australia and Russia [
3–
13]. Especially in Japan, Nagasawa et al. [
14] reported 7 fish species, i.e.,
Anguilla japonica, Silurus asotus, Acanthogobius flavimanus, Candidia temminckii, Pungtungia herzi, Rhinogobius fluviatilis, and
Rhinogobius sp., from western Japan as the fish hosts of
I. hypselobagri. Additionally they reviewed the literatures published in 1915–2013 on the host-parasite relationships of
I. hypselobagri infecting Japanese freshwater fishes. As the fish hosts of
I. hypselobagri, total 26 fish species in 10 families were thus listed in Japan [
14]. In Korea, only Chu et al. [
6] detected
I. hypselobagri from a snake head,
Channa argus. Recently, Shimazu et al. [
18] analyzed lots of
Isoparorchis spp. samples from various countries with morphological and molecular data, and they made the differential key for the identification of 4 valid species, i.e.,
I. hypselobagri, I. trisimilitubis, I. eurytremum and
I. tandani., and an undetermined species,
Isoparorchis sp. 3. Therefore, we intended to describe here the morphological characteristics for the specis identification of
Isoparorchis sp. detected in some species of fish from Yancheon (a branch stream of Gyeonghogang) in Sancheong-gun, Gyeongsangnam-do, Korea.
The fish collection site in Yangcheon is located in Saengbiryang-myeon, Sancheong-gun (Latitude: 35.36020; Longitude: 128.05824), Gyeongsangnam-do, Korea. Total 1,371 freshwater fish in 23 species (No. of fish), i.e., Pungtungia herzi (280), Acheilognathus majusculus (157), Zacco koreanus (154), Zacco platypus (152), Acheilognathus koreensis (91), Hemibarbus longirostris (70), Squalidus japonicus coreanus (62), Zacco temminckii (62), Coreoperca herzi (61), Squalidus chankaensis (51), Carassius auratus (43), Pseudogobio esocinus (39), Acheilognathus rhombeus (28), Acheilognathus yamatsutae (27), Sarcocheilichthys variegatus (23), Acanthorhodeus gracilis (20), Acanthorhodeus macropterus (19), Odontobutis platycephala (18), Channa argus (6), Sarcocheilichthys nigripinnis morii (3), Liobagrus mediadiposalis (3), Siniperca scherzeri (1), Micropterus salmoides (1), were examined for 4 years (2013–2016).
All collected fishes were transferred to the laboratory of the Department of Parasitology and Tropical Medicine, Gyeongsang National University College of Medicine, Jinju, Korea. After the identification of fish species, they were individually digested with artificial gastric juice to detect the metacercariae of digenetic trematodes. Worms of
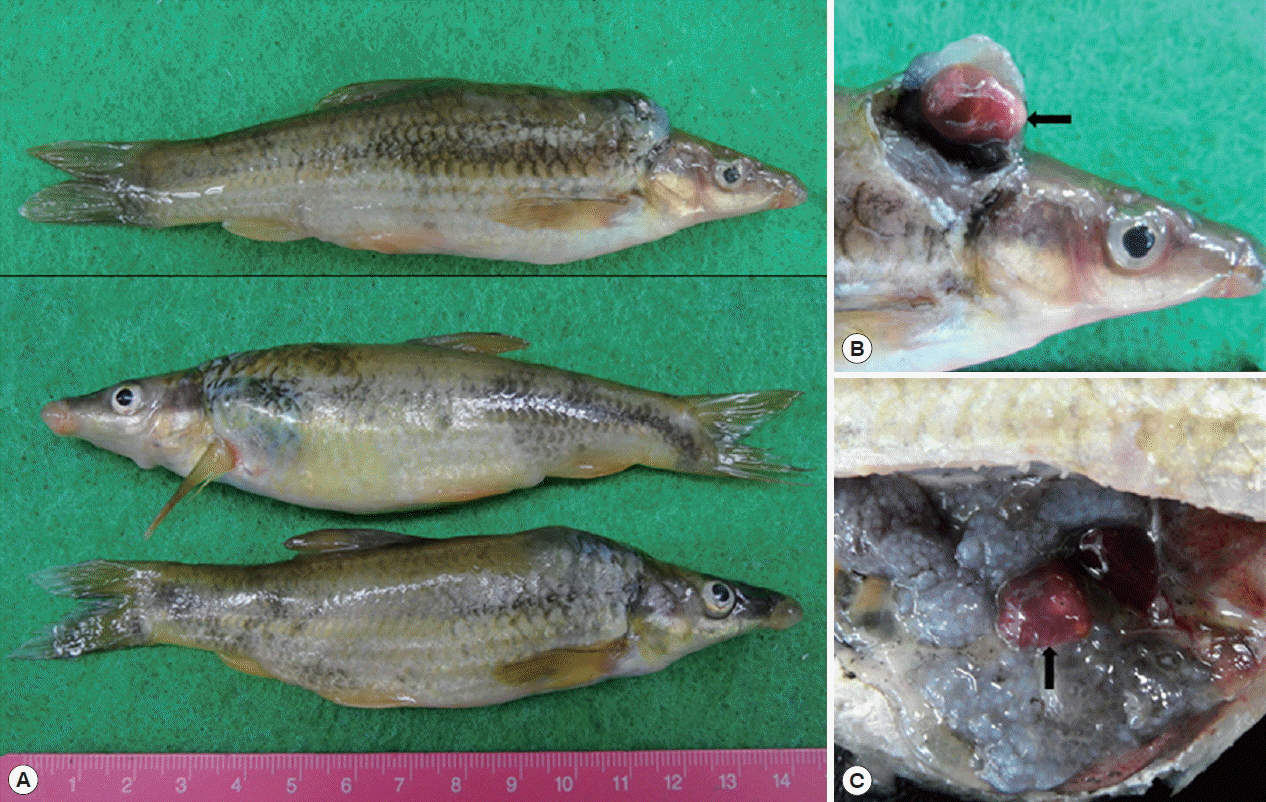
Isoparorchis sp. were found in the characteristic ink spot lesions of fish and sometimes were detected in the sediment of digested fish meat (
Fig. 1).
For the light microscopic observation, recovered flukes were fixed with 10% neutral buffered formalin under the slight pressure of cover glass. After washing with distilled water, the specimens were stained with Semichon’s acetocarmine, followed by dehydration with graded ethanol series (70%, 80%, 90%, 95% and absolute), and cleared with carbol-xylol and xylene. The specimens mounted with Canada balsam were observed under a light microscope with a micrometer (OSM-4, Olympus Co., Tokyo, Japan). Some worms were washed with 0.2 M cacodylate buffer (pH 7.2) and fixed with 2.5% glutaraldehyde for scanning electron microscopy (SEM). They were washed, dehydrated, dried, and mounted on aluminum stubs, followed by coating with gold using a JFC-1100E ion sputtering device (Jeol, Tokyo, Japan). The mounted specimens were observed using a XL-30S scanning electron microscope (Philips, Amsterdam, Netherlands) at an accelerating voltage of 15 kV.
The worms of
Isoparorchis sp. were detected in 38 (8.4%) out of 451 fish in 4 species, i.e.,
P. herzi, S. japonicus coreanus, A. koreensis and
O. platycephala, and their average density was 1.1 per fish infected. The infection status by the fish species was detailedly shown in
Table 1.
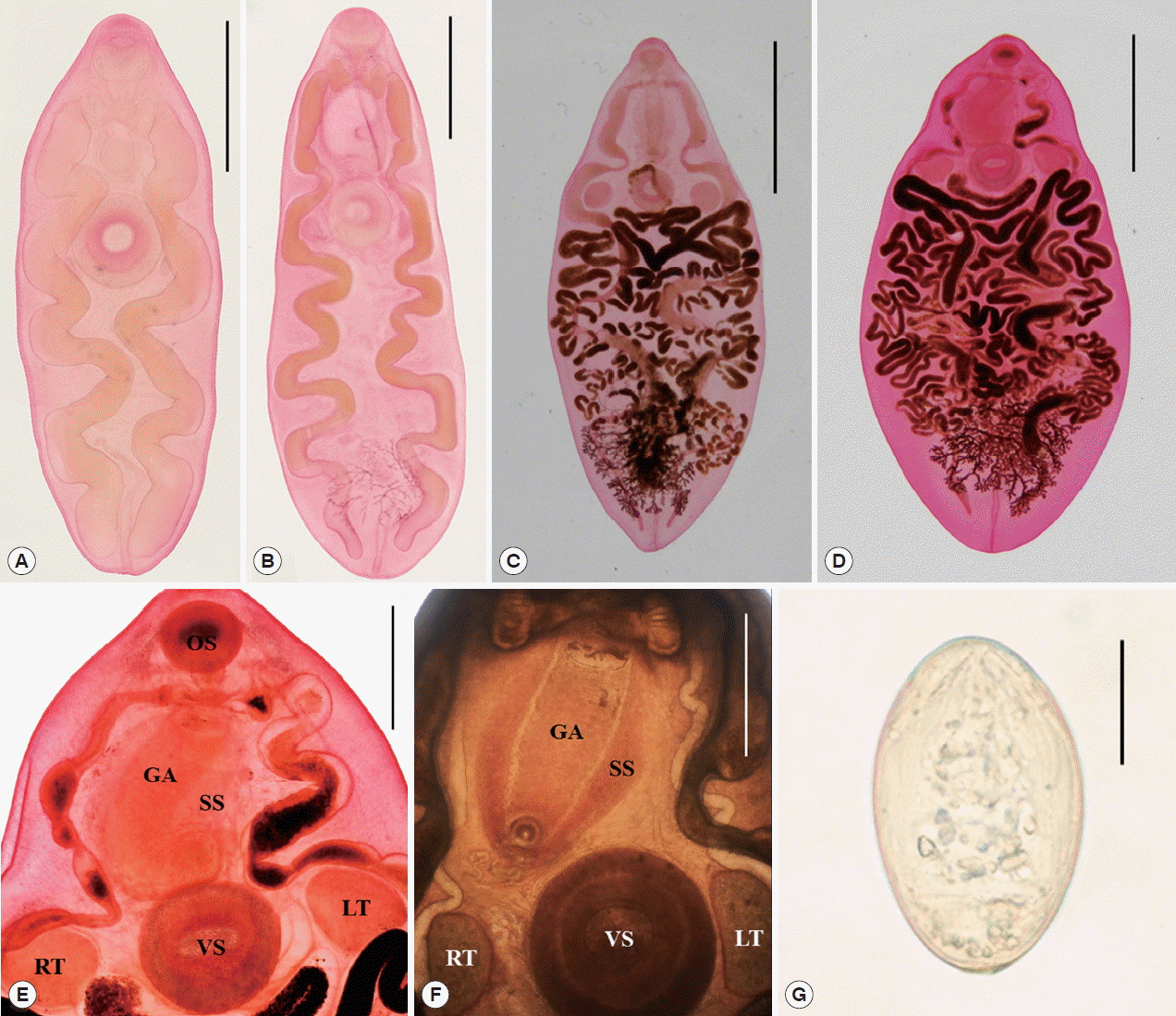
Juvenile worms (n=7) (
Fig. 2A, B): Body somewhat slender, 2.625–9.625 mm (6.639 in average) long and 1.125–4.425 (2.707) mm wide, with the attenuated forebody and the broad-elliptical hindbody, the ratio of body length to body width 2.45: 1 in average. Forebody tapering anteriorly, 0.95–2.575 (1.926) mm long, occupying 26.8–36.2 (29.0)% of body length, and hindbody almost elliptical. Oral sucker subterminal, 0.3–0.7 (0.534)×0.34–0.8 (0.607) mm. Pharynx muscular, 0.17–0.36 (0.28)×0.17–0.36 (0.283) mm. Esophagus very short, 0.07–0.25 (0.14) mm long. Intestinal ceca convoluted about 5–6 times and terminated nearly in the posterior end. Ventral sucker larger than the oral sucker, 0.56–1.25 (0.949)×0.58–1.23 (0.94) mm; the sucker width ratio 1: 1.26–1.71 (1.55). Testes round or slightly elliptical, right testis 0.45–0.64 (0.563)×0.4–0.5 (0.447) mm, left one 0.45–0.8 (0.573)×0.5–0.65 (0.573) mm, smaller than the ventral sucker, in each side of the ventral sucker. Vitellaria highly dendritic, between the last convolutions of ceca in the posteror body, observed in some specimen. Excretory pore in posterior end.
Adult worms (n=10) (
Fig. 2C, D): Body very big, 14.7–27.5 (21.6 in average) mm long and 6.075–14.0 (9.835) mm wide, with the pointed forebody and the broad-elliptical hindbody, the ratio of body length to body width 2.20: 1 in average. Forebody tapering anteriorly, 3.15–6.25 (4.848) mm long, occupying 22.4% of body length, and hindbody almost elliptical, rarely pointed. Oral sucker subterminal, 0.875–1.425 (1.03)×1.05–1.625 (1.205) mm. Pharynx muscular, 0.45–0.725 (0.55)×0.45–0.75 (0.541) mm. Esophagus very short, 0.2–0.45 (0.253) mm long. Intestinal ceca convoluted about 5–6 times and terminated nearly in the posterior end. Ventral sucker larger than the oral sucker, 1.55–2.9 (1.985)×1.65–2.95 (2.095) mm, the sucker width ratio 1: 1.56–1.87 (1.74). Testes round or slightly elliptical, right testis 0.675–2.8 (1.445)×0.85–2.575 (1.4) mm, left one 0.875–2.325 (1.41)×0.7–2.2 (1.253) mm, smaller than the ventral sucker, in each side of the ventral sucker. Seminal vesicle anterodorsal to ventral sucker. Pars prostatica posterolateral to the sinus sac. Sinus sac elliptical, large, 2.25–4.5 (3.125) by 1.5–3.0 (2.05), occupying 44.6–78.6% (64.5%) of forebody length, anterior to or overlapping ventral sucker. Genital atrium elliptical, elongated, 1.875–4.55 (2.773) by 0.625–1.175 (0.89), occupying 75.8–98.2% (88.7%) of sinus sac length (
Fig. 2E, F). Ovary dextral or sinistral. Seminal receptacle rudimental. Vitellaria highly dendritic, between the last convolutions of ceca in the posteror body. Uterus tubular, broadly occupied in the middle part of body from the just behind of the ventral sucker and testes to the anterior of vitellaria. Eggs oval, symmetrical, embryonated, 50–54 (52)×31–33 (32) μm (
Fig. 2G). Excretory pore in the posterior end.
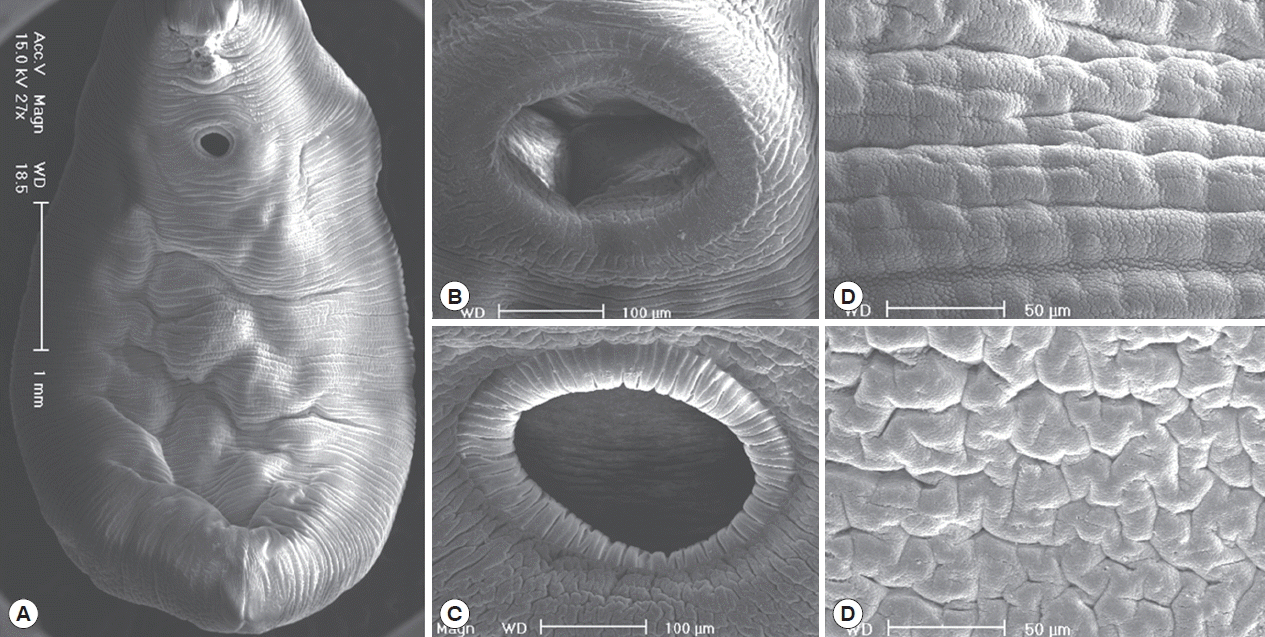
In the SEM study, adult worms were robust with a slight ventral concave and an attenuate anterior end, and they had 2 suckers and smooth body surface (
Fig. 3A). The oral sucker was slightly smaller than the ventral sucker, and they were commonly muscular and covered with the cytoplasm of striated wrinkles, but they had no sensory papillae (
Fig. 3B, C). Body surfaces in the ventral and dorsal side were commonly covered with the smooth cytoplasmic layer, but they had no tegumental spines (
Fig. 3D, E).
It has been known that members in the genus
Isoparorchis (Digenea: Isoparorchiidae) are trematodes living in the air bladder of freshwater catfishes in Asia and Australia [
1]. Since the first nomination of a new species,
Distomum hypselobagri, by Billet [
15], the generic name was emended as
Isoparorchis by Southwell [
16], and then 5 species, i.e.,
I. hypselobagri, I. trisimilitubis, I. eurytremum,
I. tandani and
I. pakistani, were described by some workers [
8,
9,
15–
17]. The debate on the species validity has long been existed in the genus
Isoparorchis and
I. hypselobagri only used to be treated as the valid one. However, in Japan, Shimazu et al. [
3] reviewed the morphological characteristics of 4 species, i.e.,
I. hypselobagri, I. trisimilitubis, I. eurytremum and
I. tandani and they asserted that Japanese specimen is
I. hypselobagri, of which the sinus organ is morphologically different those of other species. Shimazu et al. [
18] performed a molecular and morphological study with the samples of
Isoparorchis broadly collected to confirm their previous study [
3] and revise the species validity of the genus. They molecularly defined that 4 out of 5 species, i.e.,
I. hypselobagri, I. trisimilitubis, I. eurytremum and
I. tandani, are valid ones, and presented the identification key on the species of the genus
Isoparorchis [
18]. Our specimen is nearly identical with
Isoparorchis sp. 3 from India and Bangladesh and possibly Pakistan as regards the sucker (width) ratio, 1:1.7–2.0, in the identification key of Shimazu et al. [
18]. However, another morphological characters, the shape and size of sinus sac and genital atrium, are very similar with those of
I. eurytremum from Japan and Russia [
18] (
Table 2). Strictly speaking, our specimen is far from the species in the identification key of
Isoparorchis in Shimazu et al. [
18]. The sucker width ratio is slightly overlapped with
Isoparorchis sp. 3 and
I. eurytremum, and the shape and size of sinus sac and genital atrium are nearly identical with those of
I. eurytremum. Additionally, tegumental spines and sensory papillae were not observed in our specimen unlike in other trematode species in the SEM study of tegumental ultrastructures. According to the geographical distribution (Japan and Russia) and morphological similarity, our specimen is to be defined as
I. eurytremum rather than
I. hypselobagri previously described in Korea [
6]. However, we will not nominate the species name of our
Isoparorchis sp. samples until the molecular study is completely to be done with them.
The prevalence of
Isoparorchis sp., 2.2–11.4% (8.4% in average), was not so high, and the intensity of infection, 1–2 worms (1.1 in average) per fish infected, was very low in this study. Chu et al. [
6] detected only 2 juveniles in the muscle of snake head,
C. argus, purchased in a local market of Seoul. In Japan, 70.0% of 10
C. argus with 3.1 worms in average intensity (from Lake Shinji in Shimane Prefecture), [
19] and 3.2% of 2,061
Z. temmickii with 1.1 worms in average intensity (from the Shirazuna River in Nara Prefecture), were reported [
20]. In case of the latter fish species, no worm was found in small sized fish, but prevalences were steadily increased by the increase of fish size. And the seasonal prevalence was also showed and it was most highly observed in early and midwinter (December and January) [
20]. However, in this study, we did not check the seasonal prevalence in the most prevalent fish species,
P. herzi.
Conclusively, by the present study, 4 fish species i.e.,
P. herzi, A. koreensis, S. japonicus coreanus and
O. platycephala, are to be listed as the fish hosts of
Isoparorchis sp., in Korea. Additionally, the morphological characteristics of larval and adult worms of this fluke are to be described with SEM findings for the species identification of
Isoparorchis sp. from Korea. In Korea, only snake head,
C. argus, has been known as a fish host of this trematode [
6]. However, in Japan, total 26 fish species including
C. argus have been listed in the fauna of this fluke [
14]. Among 4 fish species,
P. herzi was also reported in Japan, but remaining 3 ones were not included in the Japanese list.