1. Abdolrasouli A, Amin A, Baharsefat M, Roushan A, Hemmati Y.
Moraxella catarrhalis associated with acute urethritis imitating gonorrhoea acquired by oral-genital contact.
Int J STD AIDS 2007;18:579-580
http://doi.org/10.1258/095646207781439775


2. Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. Global estimates of the prevalence and incidence of four curable sexually transmitted infections in 2012 based on systematic review and global reporting.
PLoS One 2015;10:e0143304
http://doi.org/10.1371/journal.pone.0143304



3. Sutcliffe S, Neace C, Magnuson NS, Reeves R, Alderete JF. Trichomonosis, a common curable STI, and prostate carcinogenesis--a proposed molecular mechanism.
PLoS Pathog 2012;8:e1002801
http://doi.org/10.1371/journal.ppat.1002801



4. Lee JJ, Moon HS, Lee TY, Hwang HS, Ahn MH, Ryu JS. PCR for diagnosis of male
Trichomonas vaginalis infection with chronic prostatitis and urethritis.
Korean J Parasitol 2012;50:157-159
http://doi.org/10.3347/kjp.2012.50.2.157



5. Mitteregger D, Aberle SW, Makristathis A, Walochnik J, Brozek W, Marberger M, Kramer G. High detection rate of
Trichomonas vaginalis in benign hyperplastic prostatic tissue.
Med Microbiol Immunol 2012;201:113-116
http://doi.org/10.1007/s00430-011-0205-2


6. Gardner WA Jr, Culberson DE, Bennett BD.
Trichomonas vaginalis in the prostate gland.
Arch Pathol Lab Med 1986;110:430-432.

7. Jang KS, Han IH, Lee SJ, Yoo J, Kim YS, Sim S, Ryu JS. Experimental rat prostatitis caused by
Trichomonas vaginalis infection.
Prostate 2019;79:379-389
http://doi.org/10.1002/pros.23744


10. Begley LA, Kasina S, MacDonald J, Macoska JA. The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy.
Cytokine 2008;43:194-199
http://doi.org/10.1016/j.cyto.2008.05.012



11. Penna G, Fibbi B, Amuchastegui S, Cossetti C, Aquilano F, Laverny G, Gacci M, Crescioli C, Maggi M, Adorini L. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation.
J Immunol 2009;182:4056-4064
http://doi.org/10.4049/jimmunol.0801875


13. La Vignera S, Condorelli RA, Russo GI, Morgia G, Calogero AE. Endocrine control of benign prostatic hyperplasia.
Andrology 2016;4:404-411
http://doi.org/10.1111/andr.12186


15. Parsons JK, Sarma AV, McVary K, Wei JT. Obesity and benign prostatic hyperplasia: clinical connections, emerging etiological paradigms and future directions.
J Urol 2013;189(suppl):102-106
http://doi.org/10.1016/j.juro.2012.11.029

16. Corona G, Vignozzi L, Rastrelli G, Lotti F, Cipriani S, Maggi M. Benign prostatic hyperplasia: a new metabolic disease of the aging male and its correlation with sexual dysfunctions.
Int J Endocrinol 2014;2014:329456
http://doi.org/10.1155/2014/329456



18. Teixeira L, Moreira J, Melo J, Bezerra F, Marques RM, Ferreirinha P, Correia A, Monteiro MP, Ferreira PG, Vilanova M. Immune response in the adipose tissue of lean mice infected with the protozoan parasite
Neospora caninum.
Immunology 2015;145:242-257
http://doi.org/10.1111/imm.12440



19. Margetic S, Gazzola C, Pegg GG, Hill RA. Leptin: a review of its peripheral actions and interactions.
Int J Obes Relat Metab Disord 2002;2:1407-1433
http://doi.org/10.1038/sj.ijo.0802142

20. Szyszka M, Tyczewska M, Milecka P, Jopek K, Celichowski P, Malendowicz LK, Rucinski M. Effects of leptin on leptin receptor isoform expression and proliferative activity in human normal prostate and prostate cancer cell lines.
Oncol Rep 2018;39:182-192
http://doi.org/10.3892/or.2017.6066


22. Zheng XJ, Yang ZX, Dong YJ, Zhang GY, Sun MF, An XK, Pan LH, Zhang SL. Downregulation of leptin inhibits growth and induces apoptosis of lung cancer cells via the Notch and JAK/STAT3 signaling pathways.
Biology Open 2016;5:794-800
http://doi.org/10.1242/bio.017798



25. Jung JH, Ahn SV, Song JM, Chang SJ, Kim KJ, Kwon SW, Park SY, Koh SB. Obesity as a risk factor for prostatic enlargement: a retrospective cohort study in Korea.
Int Neurourol J 2016;20:321-328
http://doi.org/10.5213/inj.1632584.292



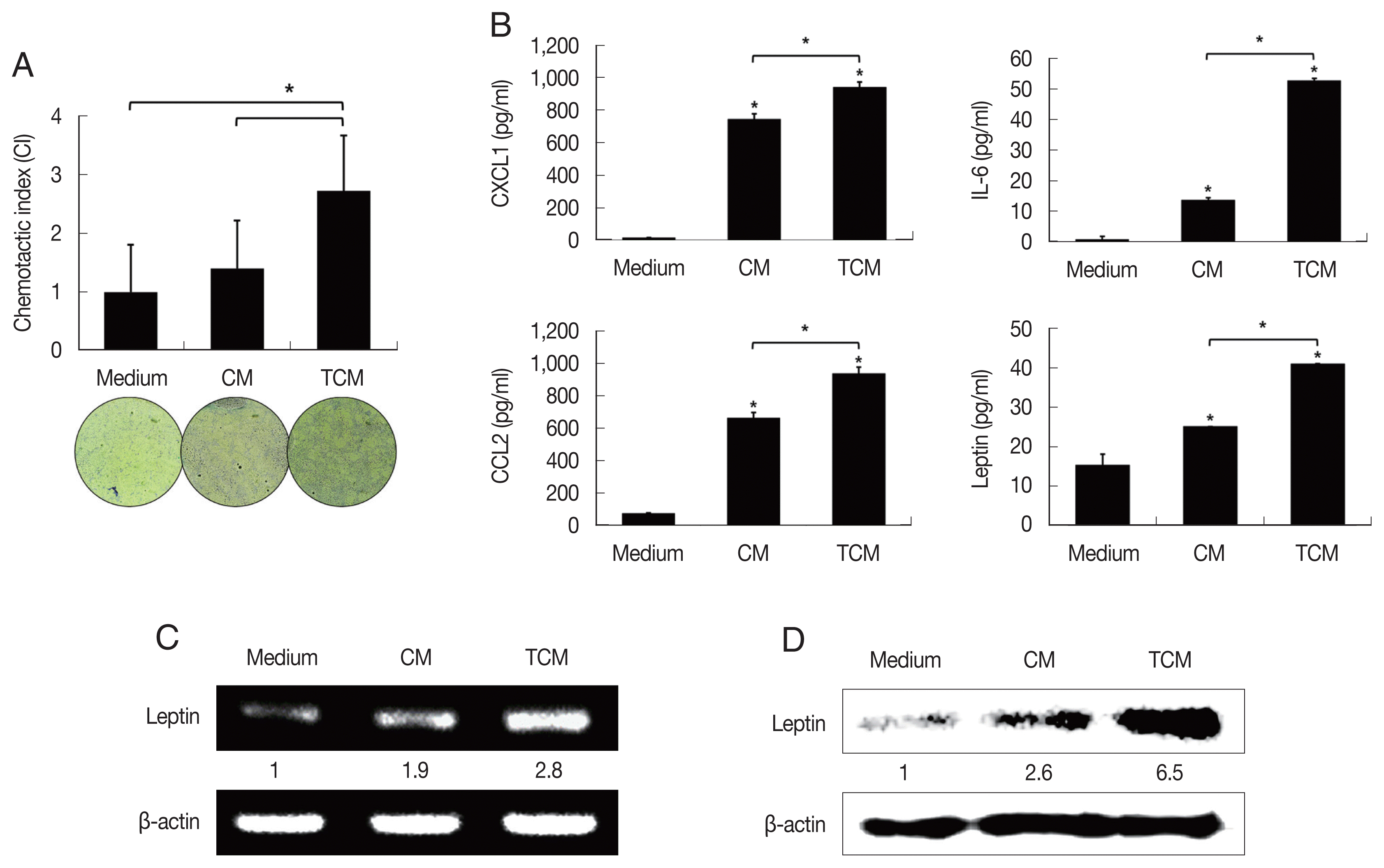
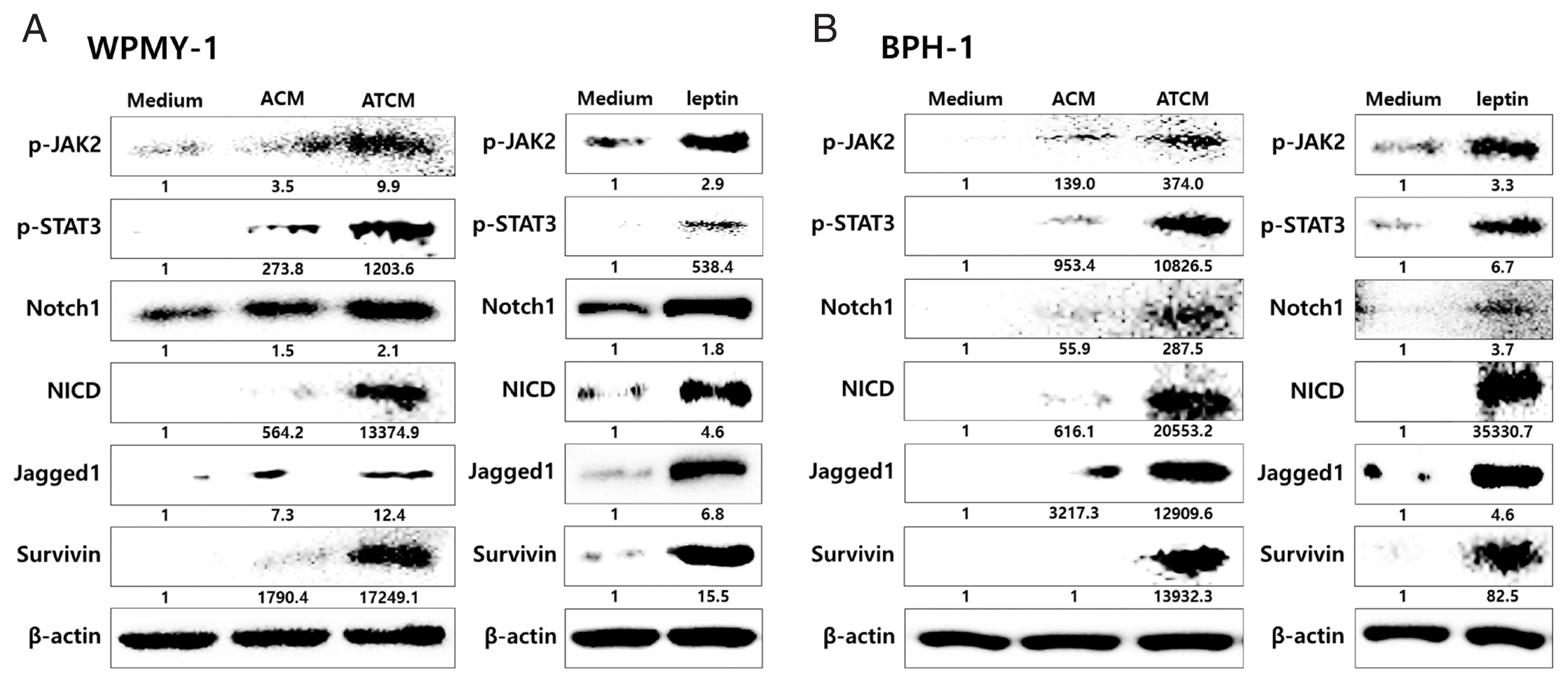
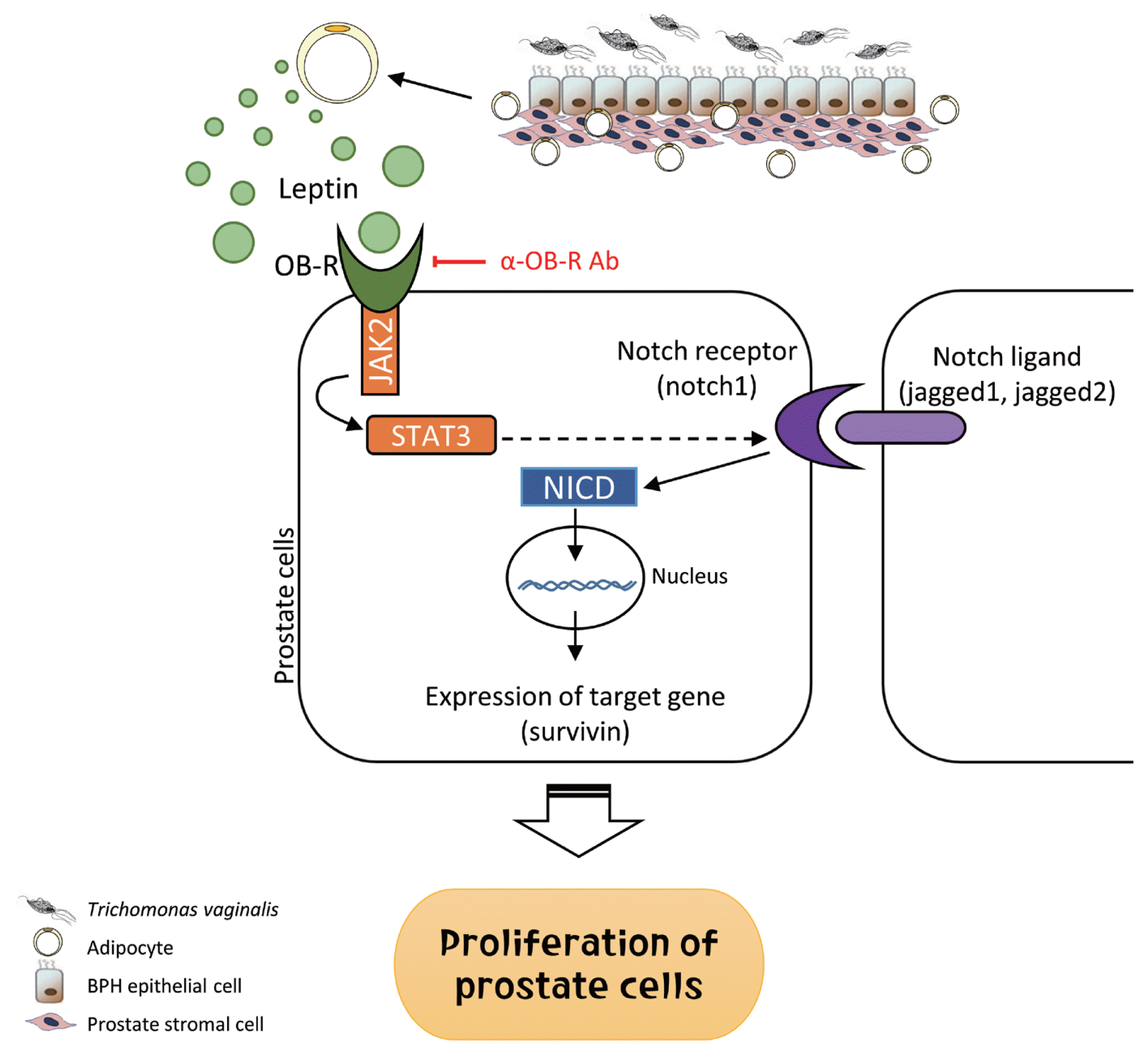
26. Kim JH, Kim SS, Han IH, Sim S, Ahn MH, Ryu JS. Proliferation of prostate stromal cell induced by benign prostatic hyperplasia epithelial cell stimulated with
Trichomonas vaginalis via crosstalk with mast cell.
Prostate 2016;76:1431-1444
http://doi.org/10.1002/pros.23227


27. Kim JH, Han IH, Kim YS, Noh CS, Ryu JS. Proliferation of prostate epithelia induced by IL-6 from stroma reacted with
Trichomonas vaginalis.
Parasite Immunol 2018;40:e12531
http://doi.org/10.1111/pim.12531


28. Chung HY, Kim JH, Han IH, Ryu JS. Polarization of M2 Macrophages by Interaction between Prostate Cancer Cells Treated with
Trichomonas vaginalis and Adipocytes.
Korean J Parasitol 2020;58:217-227
https://doi.org/10.3347/kjp.2020.58.3.217



29. Xie H, Li L, Zhu G, Dang Q, Ma Z, He D, Chang L, Song W, Chang HC, Krolewski JJ, Nastiuk KL, Yeh S, Chang C. Infiltrated pre-adipocytes increase prostate cancer metastasis via modulation of the miR-301a/androgen receptor (AR)/TGF-beta1/Smad/MMP9 signals.
Oncotarget 2015;6:12326-12339
http://doi.org/10.18632/oncotarget.3619



30. Yamashita T, Murakami T, Otani S, Kuwajima M, Shima K. Leptin receptor signal transduction: OBRa and OBRb of fa type.
Biochem Biophys Res Commun 1998;246:752-759
http://doi.org/10.1006/bbrc.1998.8689


32. Noda T, Kikugawa T, Tanji N, Miura N, Asai S, Higashiyama S, Yokoyama M. Longterm exposure to leptin enhances the growth of prostate cancer cells.
Int J Oncol 2015;46:1535-1542
http://doi.org/10.3892/ijo.2015.2845


33. Claus S, Wrenger M, Senge T, Schulze H. Immunohistochemical determination of age related proliferation rates in normal and benign hyperplastic human prostates.
Urol Res 1993;21:305-308
http://doi.org/10.1007/BF00296825


34. Harbuzariu A, Rampoldi A, Daley-Brown DS, Candelaria P, Harmon TL, Lipsey CC, Beech DJ, Quarshie A, Ilies GO, Gonzalez-Perez RR. Leptin-Notch signaling axis is involved in pancreatic cancer progression.
Oncotarget 2017;8:7740-7752
http://doi.org/10.18632/oncotarget.13946


35. Hobbs MM, Kazembe P, Reed AW, Miller WC, Nkata E, Zimba D, Daly CC, Chakraborty H, Cohen MS, Hoffman I.
Trichomonas vaginalis as a cause of urethritis in Malawian men.
Sex Transm Dis 1999;26:381-387
http://doi.org/10.1097/00007435-199908000-00003


36. Schwebke JR, Hook EW 3rd. High rates of
Trichomonas vaginalis among men attending a sexually transmitted diseases clinic: implications for screening and urethritis management.
J Infect Dis 2003;188:465-468
http://doi.org/10.1086/376558


37. Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate.
Int J Cancer 2003;107:1-10
http://doi.org/10.1002/ijc.11335


38. Siejka A, Schally AV, Barabutis N. The effect of LHRH antagonist cetrorelix in crossover conditioned media from epithelial (BPH-1) and stromal (WPMY-1) prostate cells.
Horm Metab Res 2014;46:21-26
http://doi.org/10.1055/s-0033-1349127


40. Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gómez-Reino JJ, Mera A, Lago F, Gómez R, Gualillo O. Leptin in the interplay of inflammation, metabolism and immune system disorders.
Nat Rev Rheumatol 2017;13:100-109
http://doi.org/10.1038/nrrheum.2016.209


41. Sierra-Honigmann MR, Nath AK, Murakami C, García-Cardeña G, Papapetropoulos A, Sessa WC, Madge LA, Schechner JS, Schwabb MB, Polverini PJ, Flores-Riveros JR. Biological action of leptin as an angiogenic factor.
Science 1998;281:1683-1686
http://doi.org/10.1126/science.281.5383.1683


43. Bianchi-Frias D, Vakar-Lopez F, Coleman IM, Plymate SR, Reed MJ, Nelson PS. The effects of aging on the molecular and cellular composition of the prostate microenvironment.
PLoS One 2010;5:
http://doi.org/10.1371/journal.pone.0012501

45. Battle M, Gillespie C, Quarshie A, Lanier V, Harmon T, Wilson K, Torroella-Kouri M, Gonzalez-Perez RR. Obesity induced a leptin-Notch signaling axis in breast cancer.
Int J Cancer 2014;134:1605-1616
http://doi.org/10.1002/ijc.28496


46. Guo S, Gonzalez-Perez RR. Notch, IL-1 and leptin crosstalk outcome (NILCO) is critical for leptin-induced proliferation, migration and VEGF/VEGFR-2 expression in breast cancer.
PLoS One 2011;6:e21467
http://doi.org/10.1371/journal.pone.0021467



47. Leze E, Alves-Pereira JL, Colli S, Cavalcante FS, José Sampaio F, da Fonte Ramos C. Leptin regulates proliferation and apoptosis in human prostate.
ScientificWorldJournal 2012;2012:842301
http://doi.org/10.1100/2012/842301



48. Habib CN, Al-Abd AM, Tolba MF, Khalifa AE, Khedr A, Mosli HA, Abdel-Naim AB. Leptin influences estrogen metabolism and accelerates prostate cell proliferation.
Life Sci 2015;121:10-15
http://doi.org/10.1016/j.lfs.2014.11.007


51. Cheng P, Kumar V, Liu H, Youn JI, Fishman M, Sherman S, Gabrilovich D. Effects of notch signaling on regulation of myeloid cell differentiation in cancer.
Cancer Res 2014;74:141-152
http://doi.org/10.1158/0008-5472.CAN-13-1686


52. Jarriault S, Brou C, Logeat F, Schroeter EH, Kopan R, Israel A. Signalling downstream of activated mammalian Notch.
Nature 1995;377:355-358
http://doi.org/10.1038/377355a0


53. Lipsey CC, Harbuzariu A, Daley-Brown D, Gonzalez-Perez RR. Oncogenic role of leptin and notch interleukin-1 leptin crosstalk outcome in cancer.
World J Methodol 2016;6:43-55
http://doi.org/10.5662/wjm.v6.i1.43



54. Soylu H, Acar N, Ozbey O, Unal B, Koksal IT, Bassorgun I, Ciftcioglu A, Ustunel I. Characterization of notch signalling pathway members in normal prostate, prostatic intraepithelial neoplasia (PIN) and prostatic adenocarcinoma.
Pathol Oncol Res 2016;22:87-94
https://doi.org/10.1007/s12253-015-9983-y


55. Altieri DC. Molecular circuits of apoptosis regulation and cell division control: the survivin paradigm.
J Cell Biochem 2004;92:656-663
http://doi.org/10.1002/jcb.20140


56. Ambrosini G, Plescia J, Chu KC, High KA, Altieri DC. Activation-dependent exposure of the inter-EGF sequence Leu83-Leu88 in factor Xa mediates ligand binding to effector cell protease receptor-1.
J Biol Chem 1997;272:8340-8345
http://doi.org/10.1074/jbc.272.13.8340


57. Kawasaki H, Altieri DC, Lu CD, Toyoda M, Tenjo T, Tanigawa N. Inhibition of apoptosis by survivin predicts shorter survival rates in colorectal cancer.
Cancer Res 1998;58:5071-5074.

59. Knight BB, Oprea-Ilies GM, Nagalingam A, Yang L, Cohen C, Saxena NK, Sharma D. Survivin upregulation, dependent on leptin-EGFR-Notch1 axis, is essential for leptin-induced migration of breast carcinoma cells.
Endocr Relat Cancer 2011;18:413-428
http://doi.org/10.1530/ERC-11-0075