Anticoccidial activities of a multicomplex mineral-based diet in broilers infected with Eimeria acervulina
Article information
Abstract
Poultry coccidiosis, caused by 7 Eimeria species, has a significant economic impact on the poultry industry and is managed mainly by chemotherapeutic drugs. However, alternative control measures are needed due to the emergence of drug-resistant strains. This study aimed to evaluate the anticoccidial effects of a multicomplex mineral-based diet in broilers infected with Eimeria acervulina. Broilers were fed a multicomplex mineral-based diet and infected with E. acervulina. Fecal oocyst shedding was 39.0% lower in the E. acervulina-infected broilers treated with the multicomplex mineral compared to that in untreated-infected broilers (365.7×106±45.7 versus 599.8×106±8.7, P<0.05). On day 6 post-infection, body weight gain was significantly higher in treated-infected chickens than untreated chickens (103.2±1.5% versus 94.1±1.7%, P<0.001). The lesion scores were similar between the 2 groups. Histopathological observations revealed that the width of the villi in the treated-infected chickens (286±9.5 μm) was significantly increased by 19.1% (240±10.8 μm, P<0.05) and 34.9% (212±7.3 μm, P<0.001) compared to those in the untreated-uninfected and untreated-infected groups, respectively. However, the villous height and crypt depth were similar between the untreated- and treated-infected groups. The positive effects of the dietary multicomplex mineral, including reduced fecal oocyst shedding, increased weight gain, and increased villi width, suggest its potential application in mitigating the adverse effects of Eimeria infection in both conventional and organic chicken industries.
Introduction
Coccidiosis, one of the most prevalent and severe internal parasitic diseases, is caused by 7 identified pathogens, including Eimeria acervulina, Eimeria maxima, Eimeria mitis, Eimeria necatrix, Eimeria praecox, Eimeria brunetti, and Eimeria tenella [1,2]. Additionally, the involvement of genetic variants of 3 Eimeria species, specifically, operational taxonomic units (OTU)-X, OTU-Y, and OTU-Z, was recently confirmed using next-generation sequencing [3].
When chickens orally ingest sporulated oocysts from the environment through live action, Eimeria invades the specific area in the host’s intestinal tract and undergoes a complex life cycle, producing numerous new generations of the species [2,4]. During the multiplication of Eimeria, trophozoites and merozoites disrupt the homeostasis of intestinal epithelial cells, leading to increased production losses and mortality that may vary depending on the Eimeria species; additionally, diarrhea, reduced feed efficiency, body weight loss, decreased egg production, and greater susceptibility to secondary pathogens may be observed in affected chickens [5,6]. Thus, Eimeria infection is one of the most significant and expensive diseases in the poultry industry, with prevention and treatment costs estimated to exceed 14.5 billion US dollars annually [2,7]. Anticoccidial feed additives have been used to prevent avian coccidiosis for decades and have successfully reduced economic losses. However, the emergence of drug-resistant strains has led to the need for a new alternative method for controlling avian coccidiosis [8,9].
Minerals can improve intestinal function and integrity, thereby reducing inflammation and modifying the microbiome. Thus, they play an important role in directly or indirectly affecting the host animal’s gastrointestinal environment and healthy development [5,10,11]. In Eimeria-infected chickens, Zn or Cu supplementation can improve intestinal integrity, reduce the intestinal lesion score, and mitigate fecal oocyst shedding [12]. Dietary organic and inorganic sulfur has been shown to have no or minimal effect on the growth performance of chickens challenged with a 30-fold dose of a commercial coccidiosis vaccine [13]. Generally, higher rates of mineral absorption have been described in the duodenum compared to the more distal intestines in broilers [10]. Intestinal tissue destruction in Eimeria-infected chickens causes growth retardation and malabsorption of feed and minerals [10,14]. Preparations combining several ingredients instead of a single supplement have been tested against Eimeria infections in an attempt to enhance or improve the anticoccidial effect of natural ingredients. An evaluation of the effect of trace mineral supplementation, including Zn, Cu, and Mn, in broilers infected with a commercial coccidiosis vaccine showed improved growth performance and an enhanced partial immune response to coccidiosis [15]. The anticoccidial effect of a combination of 3 natural plant products tested against E. tenella in broilers was found to be superior to that of a preparation containing 1 or 2 natural plant products [16].
This study aimed to investigate the potential use of a multicomplex mineral in mitigating adverse effects in E. acervulina-infected broilers by assessing the body weight, lesion score, fecal oocyst excretion, and histopathological findings. Our results indicate that the multicomplex mineral could be considered as a novel alternative for the prevention and management of coccidiosis in broilers.
Materials and Methods
Ethics statement
All animal maintenance and experimental procedures were performed according to the Gyeongsang National University guidelines for the care and use of experimental animals and were approved by the Institutional Animal Care and Use of Committee (GNU-201116-C0084).
Experimental design
Broilers (age, 2–3 weeks) were fed a standard diet supplemented with a multicomplex mineral 2 days before infection until the end of the experiment. The body weight gain (BWG), lesion score, fecal oocyst shedding, and histopathological findings were assessed. The experimental timeline is outlined in Supplementary Fig. S1. Two independent experiments were conducted with 734 broilers (367 broilers in each experiment).
Multicomplex mineral
A multicomplex mineral was purchased from a local company (K-mint, Jinju, Korea). For the preparation of the multicomplex mineral, iron ore was first pulverized into powder using a 100–300 mesh; subsequently, 12 g of the iron ore powder was mixed with 24 g of loess, 12 g of sulfuric acid, 5 g of citric acid, and water to make 100 g. The mixture was stirred at 50°C–80°C for 72–96 h and filtered. The filtrate was used as a multicomplex mineral in this study. A detailed description of the 20-mineral composition of the multicomplex mineral is provided in Supplementary Table S1. The composition was analyzed at the Korea Basic Science Institute, Daejeon, Korea.
Animals and infection
One-day-old Ross 308 commercial broilers were purchased from a local hatchery company (Samhwa, Hongseong, Korea), housed in wired cages at 32°C–34°C for 1 week, and maintained at room temperature (26°C–28°C) after 7 days of age. The chicks were provided with constant light throughout the experiment and raised with anticoccidial/antibiotic-free feed and water ad libitum.
The wild-type strain of E. acervulina was already developed and maintained at Gyeongsang National University, Jinju, Korea [17]. For the sporulation of fecal oocysts, fecal samples were passed through a gauze to remove debris and washed 3 times with phosphate-buffered saline (PBS) by centrifugation. The precipitates were suspended in 2.5% potassium dichromate (Daejung Chemicals and Metals, Siheung, Korea) and incubated at 150 rpm and a temperature of 25°C–28°C for 2 days for sporulation. The oocysts for infection were cleaned by flotation using 5.2% sodium hypochlorite and washed 3 times with PBS by centrifugation; subsequently, the resultant pellet containing the oocysts was resuspended in PBS.
Humane endpoint criteria were set for all animals: severe moribund animals exhibiting severe weight loss and tremors or those that were unresponsive and unaware of stimuli were euthanized immediately by atlanto-occipital dislocation. All remaining animals were euthanized by atlanto-occipital dislocation at specific time points after inoculation.
Evaluation of the BWG and sporulation rates
Male and female broiler chickens were treated with 3 different concentrations of a multicomplex mineral (1:5,000, 1:10,000, and 1:20,000), and their BWG was monitored for 6 days. Fecal samples were collected from the chickens between 5 and 7 days after infection with E. acervulina and washed 3 times with 1× PBS to remove debris and evaluate the sporulation rates. The fecal samples were added to 5,000, 10,000, and 20,000 diluted solutions of a multicomplex mineral, followed by the addition of potassium dichromate (concentration, 2.5%). Only 2.5% potassium dichromate was used in the negative control group, while the positive control group (formalin group) contained 10.0% formalin in 2.5% potassium dichromate solution. The samples were incubated for 2 days at 28°C with shaking at 150 rpm. The sporulated oocysts were counted using a McMaster counting chamber. Data are represented as the mean±SE from 3 replicates.
Evaluation of the BWG, lesion score, and fecal oocyst shedding
The BWG was individually measured on days 0, 6, and 9 after Eimeria infection. The lesion scores were determined on day 6 after infection. In brief, an approximately 15-cm-long segment of the duodenum was cut longitudinally. After careful removal of the intestinal contents, 3 blinded observers independently assigned a numerical lesion score from 0 (none) to 4 (severe) to each segment based on a previously described scoring technique [4]. Fecal samples were collected from 6 to 9 days post-infection and homogenized in a blade grinder to assess the fecal oocyst shedding. Samples were diluted in saturated NaCl, and the oocysts were counted microscopically in a McMaster counting chamber. The oocyst numbers were calculated from the average of 3 counts per sample. The total oocyst numbers were calculated as follows: oocyst count×dilution factor×(fecal sample volume ÷ counting chamber volume).
Histopathological analysis
The chickens were orally infected via gavage at 2–3 weeks of age with 1×104 or 1×105 sporulated oocysts of E. acervulina for the histopathological analysis. The control birds were orally administered the same amount of PBS. The duodenum was rapidly removed on day 6 after Eimeria infection, fixed in 10.0% neutral buffered formalin, processed routinely, and embedded in paraffin wax for sectioning. The sections (thickness, 3 μm) were cut and stained with hematoxylin and eosin. Five microscopic fields (400×) were randomly selected to measure the villous height (VH), villous width (VW), and crypt depth (CD).
Statistical analysis
Data were analyzed with a one-way analysis of variance and Dunnett’s multiple comparison test using InStat statistical software version 3 (GraphPad, Boston, MA, USA). Data are expressed as the mean±SE. Differences were considered statistically significant at P<0.05.
Results
Effect of the multicomplex mineral on BWG and sporulation
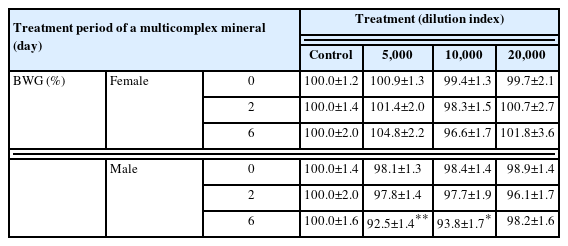
Male chickens showed a decrease in BWG under higher concentrations of multicomplex mineral, while the females did not (Table 1); the multicomplex mineral had no direct effect on the sporulation rates (Table 2). The effect of the multicomplex mineral on BWG was not significantly different between the treated female chickens and the untreated control group. Male broilers showed similar BWGs at a dilution of 20,000, but higher concentrations (5,000 and 10,000 dilutions) significantly reduced the BWG in these animals (Table 1). The sporulation rates were similar between the untreated control and treated groups; however, the 10.0% formalin treatment in the positive control group significantly reduced the sporulation rate compared to that in the untreated control group (Table 2).
Comparison of clinical symptoms in E. acervulina-infected broilers treated with the multicomplex mineral
The multicomplex mineral treatment reduced fecal oocyst production and safeguarded the BWG from E. acervulina infection but did not reduce the lesion score. At a dilution of 20,000, fecal oocyst shedding was similar between the untreated- and treated-infected groups (data not shown). Therefore, we used the 10,000 dilution for all subsequent in vivo experiments. According to the timeline in Supplementary Fig. S1, the broilers were treated with the multicomplex mineral at a dilution of 10,000 and infected with 1×104 sporulated E. acervulina oocysts. The anticoccidial effect of the treatment was assessed using 3 parameters: the BWG, lesion score, and fecal oocyst shedding (Table 3).

Comparison of clinical symptoms in broilers infected with Eimeria acervulina and treated with the multicomplex mineral
BWG was expressed relative to the body weight of the control chickens, which was taken as 100.0%. Compared to the untreated-uninfected controls (100.0±1.6%) on day 6 post-infection, BWG was significantly lower in the untreated-infected chickens (94.1±1.7%, P<0.05), but not in the treated-infected group (103.2±1.5%). The treated-infected group had a significantly higher BWG than the untreated-infected chickens (P<0.001). On day 9 post-infection, BWG was similar among the 3 groups (Table 3). Oocyst output in the treated-infected chickens (365.7×106±45.7) was significantly lower (39.0%, P<0.05) than that in the untreated-infected group (599.8×106±8.7). The mean lesion score in the untreated-infected chickens (0.4±0.2) was similar to that in the treated-infected chickens (0.5±0.2). No lesions or fecal oocysts were observed in the untreated-uninfected chickens (controls, Table 3).
Histopathological findings in the duodenum
The multicomplex mineral treatment resulted in an increase in VW but had no positive effect on the VH and CD. After infection with 1×104 sporulated oocysts, the VH was reduced by approximately 7.3% (1,494±37.9 μm) in the untreated-infected chickens and 5.5% (1,518±49.5 μm) in the treated-infected chickens compared to the untreated-uninfected controls (1,610±74.8 μm). In chickens infected with 1×105 sporulated oocysts, the VH was significantly reduced by 12.4% (1,411±40.1 μm, P<0.05) in the untreated-infected chickens and 16.8% (1,340±22.6 μm, P<0.05) in the treated-infected chickens compared to the untreated-uninfected controls (1,610±74.8 μm). The CD in the experimental chickens infected with 1×104 sporulated oocysts was increased significantly by 63.2% (395±33.5 μm, P<0.001) in the untreated-infected chickens and 64.4% (398±27.3 μm, P<0.001) in the treated-infected chickens compared to the untreated-uninfected control (242±20.2 μm). Similarly, in chickens infected with 1×105 sporulated oocysts, the CD was significantly increased by 53.3% (371±16.1 μm, P<0.001) in the untreated-infected chickens and 63.6% (396±29.8 μm, P<0.001) in the treated-infected chickens. The VW was measured in the middle area of the VH, including the CD. The VW in the experimental chickens infected with 1×104 sporulated oocysts was decreased significantly by 11.7% (212±7.3 μm, P<0.05) in the untreated-infected chickens compared to that in the untreated-uninfected controls (240±10.8 μm). However, the VW in the treated-infected chickens (286±9.5) was increased significantly by 19.1% (P<0.05) and 34.9% (P<0.001) compared to those in the untreated-uninfected and the untreated-infected chickens, respectively. Similarly, in chickens infected with 1×105 sporulated oocysts, the VW was significantly reduced by 11.7% (212±7.3 μm, P<0.05) in the untreated-infected chickens compared to the untreated-uninfected controls (240±10.8 μm). The VW in treated-infected chickens (281±10.8 μm) was significantly increased by 17.0% (P<0.05) and 32.5% (P<0.001) compared to those in the untreated-uninfected and -infected chickens, respectively (Table 4; Fig. 1).

Histological measurements in broilers infected with Eimeria acervulina and treated with the multicomplex mineral

Villous height (VH), villous width (VW), and crypt depth (CD) in the broilers. Broilers at 2 weeks of age were fed a standard diet supplemented with a multicomplex mineral (10,000 dilution) beginning 2 days before Eimeria acervulina infection until the end of the experiment. The control birds (A) were orally administered phosphate buffered saline. Chickens (B,C) were orally infected with 1×104 sporulated oocysts of E. acervulina. The duodenum on day 6 after Eimeria infection was rapidly removed and fixed in 10.0% neutral buffered formalin. The sections were stained with hematoxylin and eosin. Five microscopic fields (400×) were randomly selected to measure the VH, VW, and CD.
Significant reductions in the VH-to-CD ratio were observed in all four groups, decreasing from 44.0% to 48.0% (from 3.9±0.2 to 4.2±0.4, P<0.001) compared to the untreated-uninfected chickens (7.5±0.8) (Table 4). The VH-to-VW ratio in experimental chickens infected with 1×104 sporulated oocysts was similar between the untreated-infected group and the untreated-uninfected controls. However, the VH-to-VW ratio of the treated-infected chickens (5.4±0.2) decreased significantly by 22.2% (P<0.001) and 24.0% (P<0.001) compared to those in the untreated-uninfected (6.9±0.3) and the untreated-infected (7.1±0.2) chickens, respectively. Similarly, in chickens infected with 1×105 sporulated oocysts, the VH-to-VW ratio was similar between the untreated-uninfected control and the untreated-infected group. The VH-to-VW ratio of the treated-infected chickens (5.1±0.2) was significantly decreased by 26.1% (P<0.001) and 25.0% (P<0.001) compared with those in the untreated-uninfected (6.9±0.3) and the untreated-infected (6.8±0.3) chickens, respectively (Table 4; Fig. 1). In addition, when the VW was measured in the mid-region of the VH without CD, the VW values and VH-to-VW ratios were very similar to the above-mentioned VW values and VH-to-VW ratios obtained in the middle region of the VH with CD (data not shown).
Discussion
Eimeria infection by a single or multiple Eimeria species [17,18] destroys intestinal epithelial cells and the villi, inhibiting the absorption of feed and minerals and stunting chicken growth. Eimeria, the most common parasite found in chickens, causes avian coccidiosis, which leads to significant economic losses to the poultry industry and public health issues related to the use of anticoccidial agents [2,8]. Many reports mention the positive effects of the coccidiosis vaccine and natural products in implementing coccidiosis control strategies [2,9,19,20]. However, few studies have been conducted on the anticoccidial effects of minerals [12,15,21]. Therefore, this study investigated the potential use of a multicomplex mineral in alleviating the adverse effects of Eimeria infection in chickens. Decreased fecal oocyst shedding and increased VW, which influence the probability of coccidial infection and affect the intestinal condition and villous health, were confirmed.
In both Eimeria-infected chickens and normal animals, minerals play important roles in homeostasis, physiology, catalysis, immune response, and gut bacterial communities [10,21,22]. Studies indicate that dietary minerals have limited effectiveness in ameliorating the side effects of Eimeria infection in chickens [10,12,13]. Broilers treated with 150 ppm Cu showed significantly reduced lesion scores for E. acervulina infection in the duodenum but not for E. maxima infection in the jejunum or ileum or E. tenella infection in the cecum; alternatively, broilers fed 100 ppm Zn did not exhibit decreased intestinal lesion scores after infection with 3 different Eimeria species [12,21]. Treatments with 80 and 100 ppm Zn significantly decreased fecal oocyst production in broilers challenged with E. acervulina, but increased production in those challenged with E. maxima. In addition, treatments with 100 and 150 ppm Cu did not affect the amount of fecal oocyst production in chickens infected with 3 different Eimeria species [12]. Chickens with a mixed infection (E. acervulina, E. maxima, and E. tenella) treated with a combination of vitamin E, vitamin C, and selenium did not show reduced fecal oocyst shedding; however, the lesion scores in the treated-infected group significantly decreased in all infections compared to that in the untreated-infected group [23]. In another study, birds fed Zn and Cu and infected with four Eimeria species, including E. acervulina, E. tenella, E. maxima, and E. necatrix, had proportionally reduced oocyst shedding with reduced intestinal lesion scores [24]. The number of excreted oocysts decreased 8-fold in broilers fed a diet containing 150 mg/kg Cu and 160 mg/kg Zn compared to that in the infected group fed without Zn and Cu supplements. Similar results were observed in the current study, wherein treatment with the multicomplex mineral significantly reduced the fecal oocyst output without decreasing the lesion scores. The anticoccidial effect of a combination of multiple ingredients has been reported to be superior to that of a combination of 1 or 2 ingredients [16].
The sporulation rate of fecal oocysts is a critical factor affecting secondary Eimeria infection in a bird flock because birds can be infected only by the oral ingestion of sporulated oocysts [6]. In our study, the multicomplex mineral did not inhibit oocyst sporulation rates in fecal samples obtained after treatment and Eimeria infection (data not shown) and those supplemented with 3 different concentrations of the multicomplex mineral. Therefore, the multicomplex mineral did not affect the sporulation process of unsporulated oocysts excreted by treated and infected birds at the concentrations used in our in vitro experiments. However, several natural extracts, such as artemisinin from Artemisia annua extracts or Aloe debrana and Aloe pulcherrima leaf gels, inhibit the formation of oocyst sporulation under in vitro conditions [25,26]. In the current study, the multicomplex mineral treatment significantly reduced fecal oocyst shedding in chickens infected with E. acervulina, indicating that it exerted its anticoccidial activity only in vivo. It is speculated that the anticoccidial activity of a multicomplex mineral may be partly mediated by lessening the inflammatory response and downregulating the expression of pro-inflammatory cytokines [10] or by improving the digestibility of crude ash [13], antioxidant activities [27], or intestinal integrity [12]. Further analysis of the underlying mechanisms or metabolic pathways is necessary to determine the precise anticoccidial effects of multicomplex minerals.
Alteration in the metabolism of dietary minerals has been reported in chickens infected with Eimeria spp. [28–30]. The concentrations of Mn and Fe were decreased in the blood or serum on day 6 after E. acervulina infection compared to that in uninfected chickens [28,29]. E. tenella infection decreased the contents of Cu and Zn in the liver and blood of chickens [31]. Therefore, studies were conducted on the effect of feeding excess dietary minerals to chickens to supplement the minerals lost due to Eimeria infection. Interestingly, excess dietary Zn enhanced both the BWG rate and efficacy in E. acervulina-infected chickens but not in healthy chickens [30]. When birds fed a diet containing both 100 mg/kg Cu and 100 mg/kg Zn were infected with E. tenella, the BWG, feed conversion ratio, and lesion score improved compared to those of the infected group without the Zn and Cu supplement [31]. In other studies, the performance of healthy or infected groups fed excessive dietary Cu was similar to those of their respective controls [30]. In addition, excess dietary organic and inorganic sulfur did not affect the BWG, feed intake, or feed conversion ratio in broiler chickens challenged with 30 doses of an attenuated coccidiosis vaccine compared to those in the untreated and unchallenged groups [13]. On day 6 post-infection, chickens with Eimeria infection fed the multicomplex mineral displayed increased BWG compared to the infected group without a supplement in the present study, indicating that the multicomplex mineral positively influenced the intestinal lesions and BWG during E. acervulina infection.
Body weight changes were observed differently in healthy male and female broilers treated only with the multicomplex mineral in this study. Male and female broilers differ in growth performance due to differences in nutrient digestibility, nutrient transporter gene expression, and gut microbiota composition [32,33]. Thus, the multicomplex mineral used in this study might have different effects on body weight changes between male and female broilers; further detailed research is needed to verify this phenomenon.
Intestinal VH, VW, and CD are indicators of intestinal health and production efficiency in animals. Therefore, by measuring the ratio of VH/CD or VH/VW, it is possible to predict the ability of the intestine to digest and absorb food [34]. Eimeria infection induces a reduction in VH and an increase in CD in the chicken intestine [35]. Interestingly, VH was reported to be increased in Eimeria-infected chickens fed various diets containing Cu and Zn supplements compared to those in the infected group without Zn and Cu supplements [24]. In the current study, the multicomplex mineral did not positively affect the VH and CD. Alternatively, an increase in the VW and reduction in the VH-to-VW ratio were observed in treated-infected group compared to those in the infected group without supplements; nonetheless, the role of increased VW in Eimeria infection remains to be studied. Taken together, these results suggest that dietary minerals may influence a positive response in the gut environments of Eimeria-infected chickens. However, the excrement from broiler farms contains 4–5 different Eimeria species; hence, chickens can be infected with multiple Eimeria species simultaneously [18]. Therefore, large-scale field trials are needed to determine whether a multicomplex mineral can effectively treat multiple infections under different farm conditions.
Overall, due to the emergence of antibiotic-resistant strains and public health concerns about antibiotic residues in meat, strategies to control coccidiosis using natural substances have received increasing attention in recent years. In conclusion, multicomplex mineral-based diets significantly inhibited fecal oocyst shedding in chickens infected with E. acervulina in this study. Chickens infected with other Eimeria species suppressed fecal oocyst shedding with different sensitivities when fed the multicomplex mineral-based diet (data not shown). Thus, although the dietary multicomplex mineral has a limited role in alleviating the detrimental effects of Eimeria infection, this study illustrates its applicability for prophylactic use to control avian coccidiosis in conventional and organic chicken industries.
Notes
Author contributions
Conceptualization: Nguyen BT, Min W
Data curation: Nguyen BT
Formal analysis: Nguyen BT, Flores RA, Cammayo-Fletcher PLT
Funding acquisition: Min W
Methodology: Nguyen BT, Flores RA
Supervision: Min W
Writing – original draft: Nguyen BT
Writing – review & editing: Nguyen BT, Kim S, Kim WH, Min W
Conflict of interest
The authors declare no conflict of interest related to this study.
Acknowledgments
This work was supported by the Korea Institute of Planning and Evaluation for Technology in Food, Agriculture and Forestry (IPET) through the High-Risk Animal Infectious Disease Control Technology Development Project, funded by MAFRA (RS-2024-00399808).
Supplementary Information
Supplementary material is available with this article at https://doi.org/10.3347/PHD.24045.