Abstract
Leishmaniasis is a neglected disease and endemic in developing countries. A lack of adequate and definitive chemotherapeutic agents to fight against this infection has led to the investigation of numerous compounds. The aim of this study was to investigate the effect of RT-01, an organotellurane compound presenting biological activities, in 2 experimental systems against Leishmania amazonensis. The in vitro system consisted of promastigotes and amastigotes forms of the parasite, and the in vivo system consisted of L. amazonensis infected BALB/c mice, an extremely susceptible mouse strain. The compound proved to be toxic against promastigotes and amastigotes. The study also showed that treatment with RT-01 produces an effect similar to that treatment with the reference antimonial drug, Glucantime, in L. amazonensis infected mice. The best results were obtained following RT-01 intralesional administration (720 µg/kg/day); mice showed significant delay in the development of cutaneous lesions and decreased numbers of parasites obtained from the lesions. Significant differences in tissue pathology consisted mainly of no expressive accumulation of inflammatory cells and well-preserved structures in the skin tissue of RT-01-treated mice compared with expressive infiltration of infected cells replacing the skin tissue in lesions of untreated mice. These findings highlight the fact that the apparent potency of organotellurane compounds, together with their relatively simple structure, may represent a new avenue for the development of novel drugs to combat parasitic diseases.
-
Key words: Leishmania amazonensis, organotellurium compound, chemotherapy, leishmaniasis, antileishmanial drug
INTRODUCTION
Leishmaniasis caused by the intracellular protozoan parasite of mononuclear phagocytes
Leishmania is endemic in 88 countries [
1].
Leishmania amazonensis, a species transmitted mainly in the Amazon region, has been associated with localized cutaneous lesions, diffuse cutaneous disease, and mucosal infection [
1,
2]. The disease is neglected by the pharmaceutical industry, even though no vaccine exists, and significant side effects and signs of increasing resistance continue to occur with the use of the few effective drugs available [
3,
4].
More recently, the pharmaceutical proprieties of tellurane compounds were investigated [
5,
6]. Several tellurides show antioxidative and immunomodulating proprieties and antitumor activities [
5-
12]. Clinical trials with a tellurane compound are presently underway [
13,
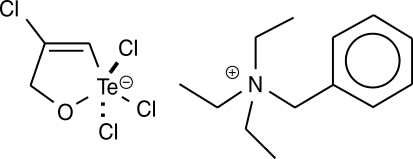
14]. The synthetic organotellurane compound RT-01 (
Fig. 1) has been previously shown to inhibit cathepsin B, a cysteine protease involved in tumor invasion, and presents a cytotoxic effect on cancer cells [
7]. In an attempt to find new leishmanial drugs, RT-01 was tested both in vitro and in vivo against
L. amazonensis. The results reported here suggest that RT-01 is effective against the flagellate and nonflagellate parasitic forms and in
L. amazonensis-infected BALB/c mice.
MATERIALS AND METHODS
Organotellurane compound (RT-01)
The organotellurane RT-01 used to evaluate the effects in vitro against
L. amazonensis and in vivo in
L. amazonensis-infected mice was prepared by the reaction of tellurium tetrachloride with propargyl alcohol in benzene followed by complexation with triethylbenzylammonium chloride, as described previously [
7,
15] (
Fig. 1). The compound was dissolved in DMSO and diluted in phosphate buffer pH 7.4, medium or saline. The final concentration of DMSO in vitro and in vivo experiments was 0.1%.
Promastigotes of
L. amazonensis (MHOM/BR/73/M2269) were cultured at 26℃ in RPMI 1640 medium (Sigma, St. Louis, Missouri, USA) supplemented with 25 µg/ml gentamicin, 2 mM L-glutamine, and 100 mM HEPES, and 10% fetal calf serum (FCS) (Cultilab, Campinas, São Paulo, Brazil). Amastigotes were isolated from active skin lesions from BALB/c mice and used immediately after isolation [
16].
Promastigotes growing in 25 cm
2 plastic flasks at 26℃ (5 × 10
6 promastigotes in total volume of 5 ml) were treated with different doses of RT-01 (0.1, 0.2, 0.3, 0.4, 0.5, 1, 2, 3, and 5 µg/ml) and their numbers were determined using a Neubauer hemocytometer at 400 × magnification [
17]. Extracellular amastigotes cultured in 25 cm
2 plastic flasks at 26℃ (5 × 10
6 amastigotes in total of 5 ml) were treated with different doses of RT-01 (0.1, 0.2, 0.4, 0.5, 0.6, 0.8, 1, and 2 µg/ml) and their viability was determined by hemocytometer counts after staining with erythrosine-B [
18]. The transformation of extracellular amastigotes into promastigotes was estimated microscopically. The stage of transformation was classified as previously described [
19].
The Ethical Committee for Animal Research of the Institute of Biology of the State University of Campinas (UNICAMP) approved the experimental protocols. Six-week-old female BALB/c mice were subcutaneously infected in the right hind footpad with 10
5 amastigotes in a volume of 20 µl. The mice were housed 7 to a cage and received sterile food and water in the Laboratory Animal Center of the Department of Parasitology, UNICAMP. RT-01 was dissolved in DMSO, diluted with saline and administered by intraperitoneal injection 30 and 15 days before infection, and 1, 15, and 30 days post-infection (PI). For each mice group (7 per group) were administered a total of 5 doses of RT-01: 180, 360, or 720 µg/kg/day. Alternatively, other groups of mice (7 per group) received the drug 3 times by intralesional injection at intervals of 10 days PI. RT-01 doses were 180, 360, or 720 µg/kg/day. Control groups of mice (7 per group) were tested with the same vehicles (DMSO and saline) without RT-01 or Glucantime (N-methyl glucamine antimonite; Rhodia, Santo Amaro, São Paulo, Brazil) at 100 mg/kg/day injected intraperitoneally for 20 days after
L. amazonensis infection used as the standard antileishmanial agent [
20]. The course of infection was monitored by measuring the increase in footpad thickness with a dial caliper, compared with the contra lateral uninfected footpad [
20]. To estimate the parasite burden in the lesions, the mice were sacrificed at the designated period, the entire infected footpads were weighed and amastigotes were recovered from the lesion, as previously described [
20]. The mice were also regularly examined to detect cutaneous ulcers, secondary lesions and secondary infection by bacteria. To perform histopathological evaluation, mouse foot tissues were fixed by immersion in 4% paraformaldehyde in 0.1 M PBS/0.1 M sucrose for 6 hr and processed for standard paraffin embedding. Tissue sections were stained with hematoxylin and eosin and examined for pathological changes under an optical microscope (Eclipse E800-Nikon, Tokyo, Japan) [
20].
All experiments were repeated at least 3 times. Three independent experiments involving 7 mice per group were performed to analyze the drug efficacy in vivo. Statistical significance between control and experimental groups were determined by the Student's t-test and the resulting data are expressed as mean ± standard error of mean (SEM).
RESULTS
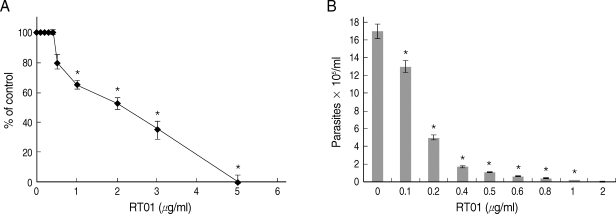
Experiments were undertaken to study the possible effects of RT-01 on promastigotes and amastigotes of
L. amazonensis. RT-01 caused a concentration-dependent loss of promastigote viability, and its toxicity effect (IC50, 24 hr) was found at approximately 2 µg/ml concentration (
Fig. 2A). Interestingly, RT-01-treated promastigotes that remained viable lost their capacity to proliferate (data not shown). Amastigotes derived from lesions were also sensitive to RT-01. Treatment of axenic amastigotes with the compound affected their viability (data not shown) and their ability to transform into promastigotes (
Fig. 2B).
Experiments were realized to determine whether RT-01 could modulate the course of infection in a cutaneous leishmaniasis model.
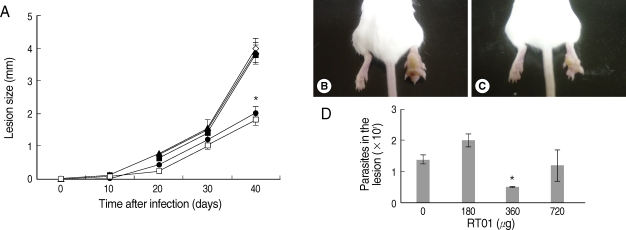
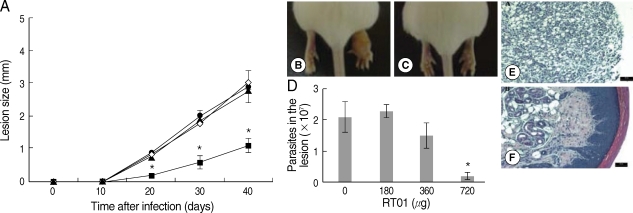
Fig. 3 demonstrates that cutaneous lesions progressively increased in size in BALB/c mice infected with
L. amazonensis. The mice failed to resolve the infection and were unable to control parasite burden in footpad lesions (
Fig. 3A, B, D). Mice treated with 360 µg/kg/day RT-01 by intraperitoneal administration showed reduced footpad thickness and approximately 3-fold fewer parasites in the footpad lesion compared to the untreated mice (
Fig. 3C, D). Mice treated with lower doses of RT-01 by intraperitoneal injection exhibited lesion progression and parasite burden similar to that of untreated mice (
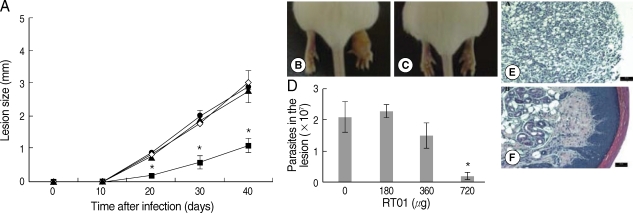
Fig. 3A, D). Treatment with 720 µg/kg/day RT-01 by intralesional administration delayed the onset of lesion development and reduced parasite tissue burden; mice presented 11-fold fewer parasites in the footpad lesion compared to the untreated mice (
Fig. 4A, C, D). We compared the effect of the standard antileishmanial drug, Glucantime, to that of RT-01 treatment. The results showed a significant decrease in the size of the lesions and parasite burden after treatment with Glucantime injected intraperitoneally, an effective route of administration of this antimonial drug for treating mice (
Fig. 3A) [
20]. A complete reduction of lesion size was not obtained, similar to the pattern observed in animals treated with RT-01 (
Figs. 3A,
4A).
Histopathology sections are shown in
Figs. 4E, F. The footpad lesions of untreated mice showed a large infiltration of infected vacuolar macrophages that replaced the normal skin tissue (
Fig. 4E). In contrast, lesions from RT-01-treated mice displayed normal skin structures; the epidermis, dermal connective tissue and sebaceous glands were histologically well-preserved (
Fig. 4F). No expressive accumulation of inflammatory cells occurred and a marked reduction in the number of macrophages and in parasitism intensity of the lesioned footpad of RT-01-treated mice was observed compared to the lesions of untreated mice (
Fig. 4F). It should be noted that RT-01 elicited no apparent toxicity; the mice neither lost weight nor did they show any alteration in gait or food consumption. In addition, long-term survival was observed in groups of mice infected and uninfected, treated 3 to 5 times with different doses of RT-01 (180 to 800 µg/kg/day).
DISCUSSION
The investigation of biological and therapeutic applications of tellurane compounds has substantially increased over the past 2 decades [
5,
6]. Organotellurane compounds present protease inhibition activity, which is specific for cysteine proteases, like papain and cathepsin B [
7,
8]. Various tellurane compounds have been described as presenting tumoricidal, antiviral, anti-protozoan parasite
Babesia rodhaini activities, and immunomodulatory properties [
7-
14,
21]. In addition to their effects, tellurium containing-compounds are characterized by low toxicity [
8,
13,
14]. RT-01, the compound studied in this work, inhibits the extracellular form of cathepsin B and showed a marked effect on human leukemia HL60 cells [
7]. To the best of our knowledge, the effects of tellurane compounds on
Leishmania have never been reported in the literature.
In the present study, direct antileishmanial activity of RT-01 on
L. amazonensis was shown. RT-01 caused a concentration-dependent loss of promastigote viability and promastigote multiplication was also hindered. The antileishmanial effects were extended to amastigotes derived from lesions. The way in which RT-01 altered the viability of
L. amazonensis in vitro was not addressed in this study. Based on the fact that tellurane compounds inhibit proteolytic enzymes, such as cysteine proteases [
7], we suggest the inhibition of parasite cysteine proteases, a virulence factor required for survival and the infection process of
Leishmania [
22,
23] by RT-01 as a hypothesis that should be investigated further.
The current study also showed that while treatment with RT-01 did not induce complete cure, it influenced the development of clinical disease in
L. amazonensis infection of an extremely susceptible mouse strain [
20,
24]. The data obtained indicate that RT-01 treatment by intralesional route generated more efficient control of parasite infection in mice than by intraperitoneal route. The best results were obtained following intralesional administration with 720 µg/kg/day, and the mice showed significant delay in the development of cutaneous lesions and a decrease in the number of the parasites obtained from the lesions that eventually developed. These results were expected, considering that a localized disease like cutaneous leishmaniasis may be affected more directly by therapeutic agents, when applied topically [
25]. The recruitment of inflammatory cells induced by RT-01 treatment in association with
Leishmania infection was not observed, but the possibility of proinflammatory effects induced by RT-01 cannot be excluded. In fact, another tellurane compound that presents a protective ability against
B. rodhaini increased the number of peripheral blood neutrophils of infected mice [
21,
26-
30].
It should be noted that RT-01 treatment produces a similar effect to that of pentavalent antimonial, Glucantime, in the lesions of mice. The antimonial compounds, the first choice treatment to all forms of leishmaniasis has the disadvantage of both toxicity (pancreatitis, cardiotoxicity, hematological disorders, and skeletal muscle toxicity) and clinical resistance where they have been used for a long time [
3,
4]. Although a toxicity profiles of RT-01 are not yet established, it can be expected that RT-01 may present similar non- or low toxicity as other organotellurane compounds due to lack of apparent toxicity of RT-01 in mice as reported in this study, excellent safety profile on clinical trials for cancer of other organotelluranes and the common tellurium-thiol chemistry of these compounds [
8,
13,
14,
21].
Evaluation of the mode of action of RT-01 may lead to improved protocols for inducing parasite clearance and the cure of leishmaniasis. Finally, these findings highlight the fact that the apparent potency of organotellurane compounds, together with their relatively simple structure, may represent a new avenue for the development of novel drugs to combat parasitic diseases.
ACKNOWLEDGEMENTS
This work was supported by the Fundação de Amparo a Pesquisa do Estado de São Paulo, Conselho Nacional de Desenvolvimento Cientifico e Tecnológico e Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil.
References
- 1. Murray HW, Berman JD, Davies CR, Saravia NG. Advances in leishmaniasis. Lancet 2005;366:1561-1577.
- 2. Grimaldi GJ, Tesh RB. Leishmaniasis of the new world: current concepts and implications for future research. Clin Microbiol Rev 1993;6:230-250.
- 3. Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis 2004;27:305-318.
- 4. Mishra JA, Saxena A, Singh S. Chemotherapy of leishmaniasis: past, present and future. Cur Med Chem 2007;14:1153-1169.
- 5. Nogueira CW, Zeni G, Rocha JBT. Organoselenium and organotellurium compounds: toxicology and pharmacology. Chem Rev 2004;104:6255-6285.
- 6. Chasteen TG, Bentley R. Biomethylation of selenium and tellurium: microorganisms and plants. Chem Rev 2003;103:1-25.
- 7. Cunha RL, Urano ME, Chagas JR, Almeida PC, Bincoletto C, Tersariol IL, Comasseto JV. Tellurium-based cysteine protease inhibitors: evaluation of novel organotellurium (IV) compounds as inhibitors of human cathepsin B. Bioorg Med Chem Lett 2005;15:755-760.
- 8. Abondanza TS, Oliveira CR, Barbosa CM, Pereira FE, Cunha RL, Caires AC, Comasseto JV, Queiroz ML, Valadares MC, Bincoletto C. Bcl-2 expression and apoptosis induction in human HL60 leukaemic cells treated with a novel organotellurium (IV) compound RT-04. Food Chem Toxicol 2008;46:2540-2545.
- 9. Sredni B, Caspi RR, Klein A, Kalechman Y, Danziger Y, Ben Ya'akov M, Tamari T, Shalit F, Albeck M. A new immunomodulating compound (AS-101) with potential therapeutic application. Nature 1987;330:173-176.
- 10. Wieslander E, Engman L, Svensjö E, Erlansson M, Johansson U, Linden M, Andersson CM, Brattsand R. Antioxidative properties of organotellurium compounds in cell systems. Biochem Pharmacol 1998;55:573-584.
- 11. Garberg P, Engman L, Tolmachev V, Lundqvist H, Gerdes RG, Cotgreave IA. Binding of tellurium to hepatocellular selenoproteins during incubation with inorganic tellurite: consequences for the activity of selenium-dependent glutathione peroxidase. Int J Biochem Cell Biol 1999;31:291-301.
- 12. Sailer BL, Liles N, Dickerson S, Chasteen TG. Cytometric determination of novel organotellurium compound toxicity in a promyelocytic (HL-60) cell line. Arch Toxicol 2003;77:30-36.
- 13. Sredni B, Xu RH, Albeck M, Gafter U, Gal R, Shani A, Tichler T, Shapira J, Bruderman I, Catane R, Kaufman B, Whisnant JK, Mettinger KL, Kalechman Y. The protective role of the immunomodulator AS101 against chemotherapy-induced alopecia: studies on human and animal models. Int J Cancer 1996;65:97-103.
- 14. Sredni-Kenigsbuch D, Shohat M, Shohat B, Ben-Amitai D, Chan CC, David M. The novel tellurium immunomodulator AS101 inhibits interleukin-10 production and p38 MAPK expression in atopic dermatitis. J Dermatol Sci 2008;50:232-235.
- 15. Zukerman-Schpector J, Camillo RL, Comasseto JV, Cunha RLOR, Caracelli I. Benzyltriethylammonium 2,2,2,4-tetrachloro-2,5-dihydro-1,2λ5-oxatellurole. Acta Cryst 2000;C56:897-898.
- 16. Barbieri CL, Giorgio S, Merjan AJ, Figueiredo EM. Glycosphingolipid antigens from Leishmania (Leishmania) amazonensis amastigotes identified by use of a monoclonal antibody. Infect Immun 1993;61:2132-2137.
- 17. Arrais-Silva WW, Collhone MC, Ayres DC, de Souza Souto PC, Giorgio S. Effects of hyperbaric oxygen on Leishmania amazonensis promastigotes and amastigotes. Parasitol Int 2005;54:1-7.
- 18. Linares E, Giorgio S, Mortara RA, Santos CX, Yamada AT, Augusto O. Role of peroxynitrite in macrophage microbicidal mechanisms in vivo revealed by protein nitration and hydroxylation. Free Rad Biol Med 2001;30:1234-1242.
- 19. Lemestre JP, Sereno D, Daulouede S, Veyret B, Brajon N, Vincendeau P. Leishmania spp.: nitric oxide-mediated metabolic inhibition of promastigote and axenically growth amastigote form. Exp Parasitol 1997;86:58-68.
- 20. Arrais-Silva WW, Pinto EF, Rossi-Bergmann B, Giorgio S. Hyperbaric oxygen therapy reduces the size of Leishmania amazonensis-induced soft tissue lesions in mice. Acta Trop 2006;98:130-136.
- 21. Rosenblatt-Bin H, Klein A, Sredni B. Antibabesial effect of the immunomodulator AS101 in mice: role of increased production of nitric oxide. Parasite Immunol 1996;18:297-306.
- 22. Matlashewski G. Leishmania infection and virulence. Med Microbiol Immunol 2001;190:37-42.
- 23. Mottram JC, Souza AE, Hutchison JE, Carter R, Frame MJ, Coombs GH. Evidence from disruption of the lmcpb gene array of Leishmania mexicana that cysteine proteinases are virulence factors. Proc Natl Acad Sci USA 1996;93:6008-6013.
- 24. Barral-Netto M, Cardoso SA, Barral A. Different patterns of disease in two inbred mouse strains infected with a clone of Leishmania mexicana amazonensis. Acta Trop 1987;44:5-11.
- 25. Macharia JC, Bourdichon AJ, Gicheru MM. Efficacy of Trypan: a diminazene based drug as antileishmanial agent. Acta Trop 2004;92:267-272.
- 26. Frei GM, Kremer M, Hanschmann KM, Krause S, Albeck M, Sredni B, Schnierle BS. Antitumour effects in mycosis fungoides of the immunomodulatory, tellurium-based compound, AS101. Br J Dermatol 2008;158:578-586.
- 27. Hayun R, Shpungin S, Malovani H, Albeck M, Okun E, Nir U, Sredni B. Novel involvement of the immunomodulator AS101 in IL-10 signaling, via the tyrosine kinase Fer. Ann N Y Acad Sci 2007;1095:240-250.
- 28. Kalechman Y, Gafter U, Da JP, Albeck M, Alarcon-Segovia D, Sredni B. Delay in the onset of systemic lupus erythematosus following treatment with the immunomodulator AS101: association with IL-10 inhibition and increase in TNF-alpha levels. J Immunol 1997;159:2658-2667.
- 29. Sredni B, Kalechman Y, Albeck M, Gross O, Aurbach D, Sharon P, Sehgal SN, Gurwith MJ, Michlin H. Cytokine secretion effected by synergism of the immunomodulator AS101 and the protein kinase C inducer bryostatin. Immunology 1990;70:473-477.
- 30. Shohat M, Hodak E, Sredni B, Shohat B, Sredni D, David M. Cytokine profile of patients with mycosis fungoides and the immunomodulatory effect of AS101. Acta Derm Venereol 2001;81:255-257.
Fig. 1Chemical structure of RT-01. The empirical formula is C13H22N+·C3H3Cl4OTe-.
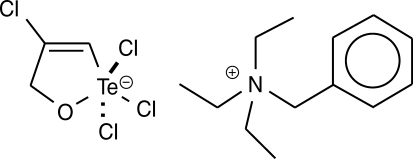
Fig. 2RT-01 toxicity for L. amazonensis. (A) Promastigotes were exposed to various concentrations of RT-01 for 24 hr at 26℃. The percentage control values were calculated by dividing promastigote numbers in the presence of RT-01 with promastigote numbers in the absence of RT-01 and multiplying by 100. (B) Extracellular amastigotes were exposed to various concentrations of RT-01 for 48 hr at 26℃. The transformation of amastigotes into promastigotes was estimated by counting promastigotes. The data shown represent the mean ± SEM. This experiment is representative of 5 independent repeats (*P < 0.01 in comparison to untreated parasites).
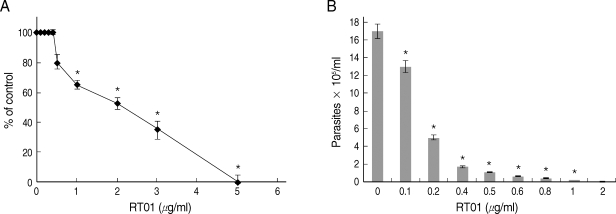
Fig. 3Follow-up of BALB/c mice infected with L. amazonensis after intraperitoneal RT-01 treatment. Mice (7 per group) were infected with 105 amastigotes. RT-01 (180 µg/kg/day; ▴), (360 µg/kg/day; •) or (720 µg/kg/day; ▪) were administered by intraperitoneal injection 30 and 15 days before infection, and day 1, 15, and 30 post-infection (PI). Glucantime (100 mg/kg/day) were administered by peritoneal injection at day 20 PI (□). Untreated mice were injected with vehicles (DMSO and saline) without RT-01 (◇). (A) Lesion size is expressed as the difference in size between the infected and contralateral non-infected footpads. (B) Appearance of a typical footpad lesion of an untreated mouse at day 40 PI. (C) Appearance of a footpad lesion of a 360 µg/kg/day RT-01-treated mouse at day 40 PI. (D) Parasite burden in infected footpad lesions from untreated mice and RT-01-treated mice at day 40 PI. This experiment is representative of 3 independent repeats (*P < 0.05 in comparison to control mice).

Fig. 4Follow-up of BALB/c mice infected with L. amazonensis after intralesional RT-01 treatment. Mice (7 per group) were infected with 105 amastigotes. RT-01 (180 µg/kg/day; ▴), (360 µg/kg/day; •) or (720 µg/kg/day; ▪) were administered 3 times at intervals of 10 days after infection. Untreated mice were injected with vehicles (DMSO and saline) without RT-01 (◇). (A) Lesion size is expressed as the difference in size between the infected and contralateral noninfected footpad. (B) Appearance of typical footpad lesion of untreated mouse at day 40 PI. (C) Appearance of footpad lesion of 720 µg/kg/day RT-01-treated mouse at day 40 PI. (D) Parasite burden in infected footpad lesion from untreated mice and RT-01-treated mice at day 40 PI. (E) Photomicrograph of the histological section of a footpad lesion from untreated mice at day 40 PI. (F) Photomicrograph of the histological section of a footpad lesion from 720 µg/kg/day RT-01-treated mouse at day 40 PI. Figs. (E) and (F) are hematoxylin and eosin stained. This experiment is representative of 3 independent repeats (*P < 0.05 in comparison to control mice).