Abstract
This study investigated whether trinitroglycerine (TNG) as nitric oxide (NO) releasing agent had anti-leishmanial effects and mediated pathology in BALB/c mice infected with Leishmania major. Cutaneous leishmaniasis (CL), a zoonotic infection caused by leishmania protozoa is still one of the health problems in the world and in Iran. NO is involved in host immune responses against intracellular L. major, and leishmania killing by macrophages is mediated by this substance. Moreover, application of CL treatment with NO-donors has been recently indicated. In our study, TNG was used for its ability to increase NO and to modify CL infection in mice, in order to evaluate NO effects on lesion size and formation, parasite proliferation inside macrophages, amastigote visceralization in target organs, and NO induction in plasma and organ suspensions. Data obtained in this study indicated that TNG increased plasma and liver-NO, reduced lesion sizes, removed amastigotes from lesions, livers, spleens, and lymph nodes, declined proliferation of amastigotes, hepatomegaly, and increased survival rate. However, TNG reduced spleen-NO and had no significant effects on spelenomegaly. The results show that TNG therapy reduced leishmaniasis and pathology in association with raised NO levels. TNG had some antiparasitic activity by reduction of positive smears from lesions, livers, spleens, and lymph nodes, which could emphasize the role of TNG to inhibit visceralization of L. major in target organs.
-
Key words: Leishmania major, BALB/c mouse, nitric oxide, trinitroglycerin, Iran
INTRODUCTION
Leishmaniasis is a parasitic disease with many distinctive manifestations, ranging from a self-healing cutaneous leishmaniasis (CL) to a lethal visceral (VL) form [
1]. CL caused by
Leishmania major and
Leishmania tropica is characterized by a skin ulcer which heals spontaneously, leaving a scar [
2]. The clinical characteristics of CL vary according to the
Leishmania species and the host's immune responses [
3]. The disease is considered as a major public health problem causing morbidity and mortality in more than 80 countries [
4], and it is widespread through some regions in Iran [
2].
Immune responses during leishmaniasis include antibodies, cytokines, immune cells, mediators, and acute phase proteins [
5]. In CL, various phagocytes predominant in the skin, including neutrophiles, macrophages (Mф), and dendritic cells, play distinct roles for the host's immune responses [
6]. In experimental infection of
L. major in inbred mice, the immune response is shown to be host genotype dependent. BALB/c mice develop severe lesions and are susceptible to the
L. major model with early T-helper-2 (Th2) responses, whereas the resistance to leishmania is conferred by Th1 type responses [
6,
7].
In human and experimental CL, development of protective immunity is dependent on the activation of infected Mф to express inducible nitric oxide synthase (iNOS), the formation of nitric oxide (NO), and elimination of the parasite via the NO pathway [
8]. NO is an effector mechanism against the intracellular
L. major parasites which is produced in large amounts in murine Mфs. In contrast, Th2 responses limit the Th1 functions, which deactivate Mф and NO production helping growth of intracellular parasites and disease progression [
6,
7,
9].
Multiple biological functions have been described for NO as a signaling, regulating, or cytotoxic molecule [
10]. Mф play a dual role in leishmaniasis; it serves as potentially safe habitats for the parasite, and when activated, they produce NO as a potent molecule against intracellular
Leishmania [
11]. Both endogenous and exogenous NO inhibits the development of intracellular and extracellular protozoa [
11-
13]. This NO acts as a key messenger and a potent biological mediator in pathological processes [
14], whereas high levels of inducible NO can exert some beneficial effects [
12].
NO plays a pivotal role as a leishmanicidal agent in mouse Mфs [
11,
12,
15]. However, the cellular and molecular mechanisms whereby NO exerts its cytotoxic activity are not yet well described. Recent studies found that apoptotic processes and several targets in organisms may be affected by NO [
8,
15,
16]. However, both in vitro and in vivo immunological studies indicate that NO radicals within leishmania lesions could reduce the parasite number [
17].
There are many therapeutic assays for CL, including surgical removal, thermotherapy, cryotherapy, electrotherapy, laser, topical treatments, intralesional or systemic injection, and immunotherapy [
18]. However, to date, there is no safe, cheap, and effective method for CL treatment [
19]. Current therapies for CL are unsatisfactory due to drug resistance, lack of efficacy, toxicity, route of administration, prolonged treatment, and high cost. Therefore, there is a requirement for the development of novel drug targets for CL treatment [
20]. Chemotherapeutic cure is largely dependent upon the development of an effective immune response that activates Mф to produce toxic mediators to kill the amastigotes [
7]. Interestingly, the treatment of CL with NO-releasing drugs has been recently approached and the effect of NO on parasite has been established [
12]. In CL, NO-generating creams applied topically to the lesions, which revealed a modest efficacy in mouse models, whereas they were able to cure human patients [
8].
Trinitroglycerin (TNG) was the first to be described as antianginal drug, and it is still used in the treatment of heart failure [
8], which is known to be an exogenous donor of NO [
21]. TNG is a substance that is metabolized to NO in the cells and its action is not dependent on the endothelium derived NO and may inhibit the catalytic activity of
Leishmania [
14]. Since, TNG has been used safely for over a century to treat medical conditions [
21], this investigation was carried out to clarify whether TNG as NO-releasing agent had anti-leishmanial effects and mediated pathology in mice infected with
L. major in vivo.
MATERIALS AND METHODS
Animals
Female inbred BALB/c mice (supplied by the Karaj Laboratory Animal Unit, Pasteur Institute of Iran) were used in this study. The initial body weight 18.2 ± 1.3 g (mean ± standard error of mean, SEM) and mice were housed at room temperature (20-23℃) on a 12-hr light and 12-hr dark cycle, with unlimited access to food and tap water. Experiments with animals were done according to the ethical standards formulated in the Declaration of Helsinki, and measures taken to protect animals from pain or discomfort. It has been approved by institutional ethical review board (Ethical Committee of the Pasteur Institute of Iran), in which the work was done.
In vitro cultivation of L. major
The
L. major used in this study was the standard strain MRHO/IR/75/ER. The infectivity of the parasites was maintained by regular passage in susceptible BALB/c mice. The parasites were cultured in the RPMI 1,640 medium supplemented with 10% fetal bovine serum (FBS) as determined by Nahrevanian et al. [
5]. Under these culture conditions, the stationary phase of parasite growth was obtained in 6 days.
Promastigotes of L. major were harvested from culture media, counted and used to infect BALB/c mice. The base of the tail was injected intradermally with inoculums of 2 × 106 promastigotes. The animal experiments were performed once in 2 groups considering time, budget, and long-period monitoring of animals according to the ethical issues for sample size and replication in order to protect animals from further pain or discomfort. It has been approved by institutional ethical review board in which the work was done.
Preliminary experiments
Preliminary experiments were applied in vitro on cultivated promastigotes and in vivo on uninfected BALB/c mice in order to optimize TNG dose and its toxicity. The optimal dose of TNG was selected to apply in infected animals according to the maximum efficacy and the minimum toxicity.
In vivo injection of TNG as a NO-donor
The total number of animals used in this experiment was 20 BALB/c mice divided into 2 groups, both were infected with L. major. The first group was injected with drug vehicle (normal saline) as the control and the TNG-treated was indicated as the test group. TNG (200 µg) was injected for its ability to increase NO and control animals were injected with normal saline subcutaneously (s.c.) for 15 days, starting from week 6 after infection in both groups.
Assessment of pathology
Measurement of lesion size
Lesions at the base of the tail was measured for its size at every other day after inoculation by a digital caliper (Chuan Brand, Beijing, China) in 2 diameters (D and d) at right angles to each other, and the size millimeters (mm) was determined according to the formula: S = (D + d)/2 [
22].
Microscopical examinations and smear preparation
The clinical diagnosis was confirmed by laboratory demonstration of the parasite in the lesions by making stained smears at the end of the experimental period as described previously [
5]. Briefly, lesions were cleaned with ethanol and punctured at the margins with a sterile lancet and exudative material was smeared and stained. Impression smears were prepared from the liver, spleen, and lymph nodes by placing a small piece of tissue between 2 glass slides and pushing them in different directions. Smears were air dried, fixed by methanol, and stained with Giemsa for examination of amastigotes (leishman bodies) as an index of visceralization by light microscopy.
Measurement of proliferation in amastigotes
The proliferation of parasites was evaluated by counting amastigotes inside Mф on Giemsa-stained lesion smears at the end of the experimental period as established by authors [
5].
Assessment of degree of hepato-splenomegaly
Entire livers and spleens were removed post-mortem at the end of the experimental period from mice and the degree of hepatomegaly and splenomegaly was determined as previously described [
5].
Body weight
Body weight was measured initially and at different times of experiment using a top pan balance (OHAUS Scale Corp., Pine Brook, New Jersey, USA).
Preparation of sera
Serum was prepared from blood taken by 10 ml insuline syringe from mice terminally anaesthetized by inhalation of diethyl ether. Serum was prepared by centrifuging blood at 1,500 g for 10 min, collected and stored at -70℃ until assayed with Griess Microassay [
23].
The Griess reaction was adapted to assay nitrate as described previously [
23]. Briefly, standard curves for nitrate (Sigma, St. Louis, Missouri, USA) were prepared and samples (50 µl plasma and 100 µl tissue suspension) was added to Griess reagent and proteins subsequently precipitated by 50 µl 10% trichloroacetic acid (Sigma). Tube contents were vortex-mixed, centrifuged, and supernatants transferred to a 96-well flat-bottomed microplate, absorbances read at 520 nm using a microplate reader, and finally values calculated from standard calibration plots.
All values are presented as the mean ± SEM for groups of 'n' samples. The significance of differences was determined by Analysis of Variances (ANOVA) and Student's t-test using Graph Pad Prism Software (Graph Pad, San Diego, California, USA).
RESULTS
NO production in plasma and tissues
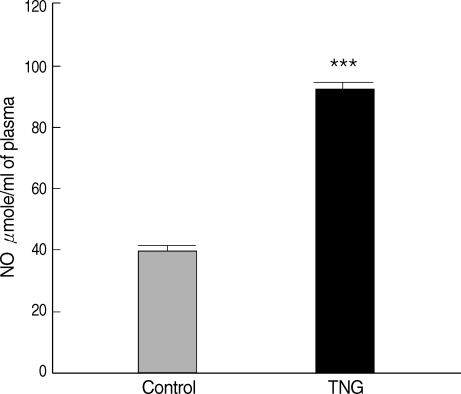
Plasma NO levels were measured for control and TNG groups. The results indicated that production of NO increased in
L. major-infected BALB/c mice treated with TNG (test) when compared with control. TNG as the NO donor showed their ability to elevate (
P < 0.001) NO in treated animals (
Fig. 1).
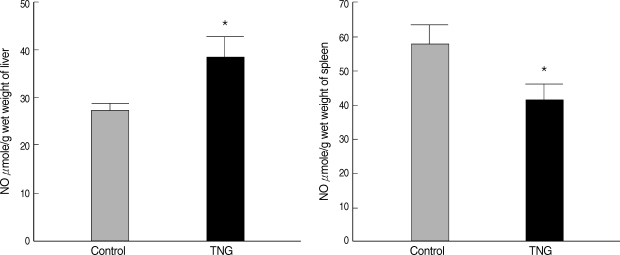
NO production was measured in suspensions of the liver and spleen of 2 control and TNG groups. There were significant increases (
P < 0.05) in the liver NO after inoculation with TNG as a putative NO donor. However, NO production in the spleen indicated some decreases (
P < 0.05) in TNG group when compared with control animals (
Fig. 2).
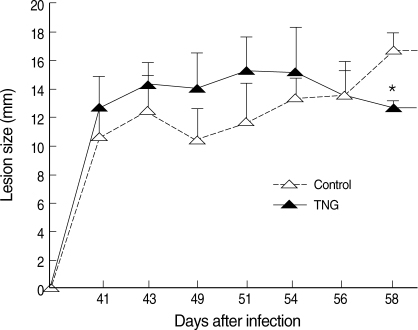
Healing of CL lesions was studied by measurement of lesion sizes in both control and TNG groups. TNG showed anti-leishmanial activity, as this drug reduced the lesion sizes (
P < 0.05) starting from week 8 after infection (
Fig. 3).
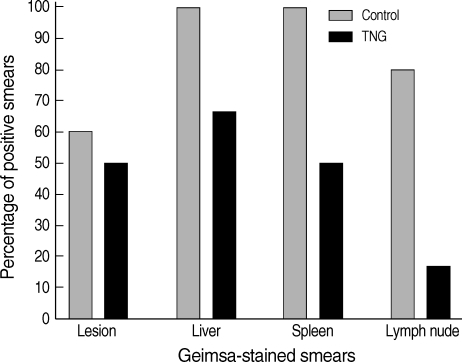
Percentages of positive Giemsa-stained smears were determined in the lesions, liver, spleen, and lymph nodes of both control and TNG groups of mice. TNG showed anti-leishmanial activity by reducing the number of amastigotes in the smears. TNG decreased percentage of positive smears in CL lesions from 59% (control) to 49% (TNG), liver smears from 98% (control) to 64% (TNG), and a sharp reduction was observed in positive smears of the spleen from 98% (control) to 49% (TNG) and lymph nodes from 78% (control) to 15% (TNG) (
Fig. 4).
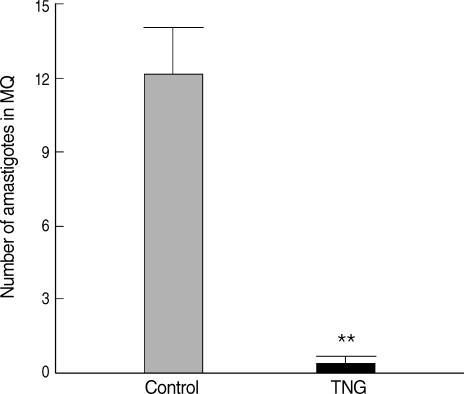
Comparative proliferation of amastigotes inside Mф was made by observation of Giemsa-stained smears of CL lesions in both groups. Putative efficacy of TNG was evidenced to reduce parasite proliferation inside Mф (
P < 0.01) in TNG as compared with that in control group (
Fig. 5).
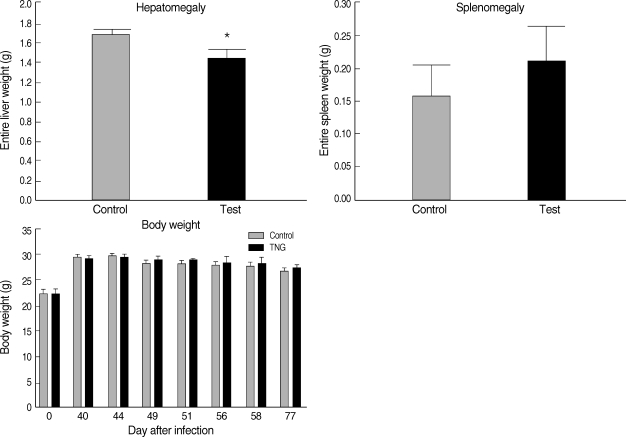
Hepatomegaly, splenomegaly, and body weight were evaluated in 2 groups of control and TNG as pathophysiological consequences of the disease in infected mice. However, in the TNG group, there was a reduction in the degree of hepatomegaly by comparing with the control group; no significant pathological effect was seen by the TNG treatment on splenomegaly and body weight (
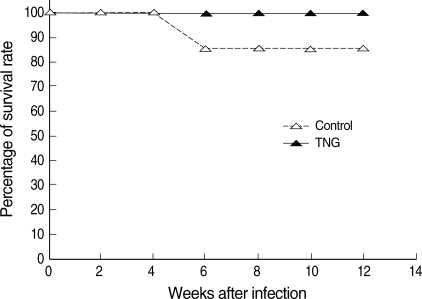
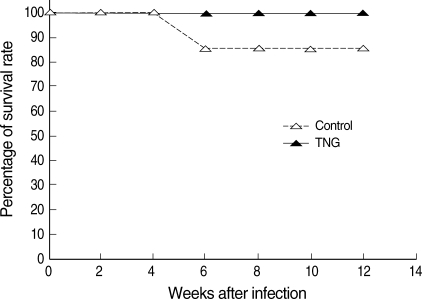
Fig. 6). In addition, the TNG treatment increased the survival rate from 85.7% to 100% at a final stage of experiment (
Fig. 7).
DISCUSSION
This is a novel idea to test endemic Iranian strain of L. major and evaluate the anti-leishmanial effects of TNG as the NO releasing agent and its pathophysiological consequences on BALB/c mice infected with Leishmania parasites in a single study. The literature on effects of NO in leishmania has been contradictory; therefore, this confusion is presumably due in part to the heterogeneous nature of leishmania, but also the differences in experimental methods. In this novel study, we have sought to establish the importance in this laboratory of using standard strain of L. major in BALB/c mice, given the general agreement in the literature that both can influence the progress of the infection. Therefore, this investigation has clarified more NO hypothesis against intracellular leishmania parasites, and it has been determined whether NO has anti-leishmanial effects and mediated pathology in BALB/c mice infected with L. major MRHO/IR/75/ER, a prevalent strain of CL in Iran. NO donor, TNG was used for its ability to increase NO and to modify leishmania infection in susceptible BALB/c mice.
Analysis of data resulted from this study revealed an association between NO levels after TNG therapy with the evolution of the disease, which had an effect on pathological signs of
L. major infected BALB/c mice. The modulation of NO was also able to modify these clinical signs and could affect the proliferation of amastigotes inside Mф, lesion sizes, degree of hepatomegaly and splenomegaly, and presence of amastigotes in the smears of lesions, livers, spleens and lymph nodes. These alterations may depend on the NO donation, dose and number of inoculation, and innate tolerance variability of hosts which were previously published in murine intracellular malarial parasites [
24,
25], and it was clarified more in such murine intracellular leishmania parasites as well.
In this study, the liver, spleen, and lymph nodes were studied as target organs and evaluated for anti-leishmanial effects of TNG and mediated pathology in vivo. The data obtained in the present study indicated that TNG-released NO might be involved in the leishmanicidal activity against
L. major. However, TNG increased the plasma and liver NO (
Figs. 1,
2), reduced lesion sizes (
Fig. 3), removed amastigotes from lesions, livers, spleens, and lymph nodes (
Fig. 4), declined proliferation of amastigotes (
Fig. 5), hepatomegaly (
Fig. 6), and increased survival rate (
Fig. 7), but it reduced spleen NO and had no significant effects on spelenomegaly. It seems that the liver and spleen play different roles in leishmaniasis via NO pathway; the liver may storage some extra NO which was produced by different cells after a systemic induction. However, NO production is proposed to be inhibited in the spleen by
L. major infection in BALB/c mice, when compared with naïve animals as confirmed by the author's previous publication [
5].
TNG had anti-leishmanial activity by reduction positive smears from the lesion, liver, spleen, and lymph nodes, which could emphasize the role of TNG to inhibit visceralization of
L. major in the target organs of infected susceptible BALB/c mice. A partial role for NO is highlighted here, which is in accordance with published reports describing that reactive nitrogen radicals represent the main mechanism in the elimination of parasites from the body [
7,
17], and NO donors have been used successfully in the treatment of CL in animal models [
5] or as a topical therapy in human CL [
21].
Consequently, it is conceivable that the protection induced by the NO donor against L. major may be attributable partly or entirely to effects other than the concurrent increase in NO produced by TNG. TNG therapy reduced leishmaniasis and pathology in association with raised plasma NO. These results demonstrated that TNG is well recognized as an exogenous donor of NO and has been used safely for over a century to treat medical condition; it also has potential therapeutic effects against CL and could be used for its leishmaniacidal activity.
ACKNOWLEDGEMENTS
This work was granted for a project number 318 by Pasteur Institute of Iran. We thank Dr. N. Namvar Asl from Karaj Laboratory Animal Unit, Pasteur Institute of Iran for kind providing of animals.
References
Fig. 1NO production in plasma of control and TNG-treated BALB/c mice. NO levels in plasma of control (normal saline) and TNG groups of L. major infected BALB/c mice were assayed by Griess Microassay at the end of the experimental period. Significance of differences (***P < 0.001) was determined by an unpaired Student's t-test using Graph Pad Prism (n = 10 mice per group).
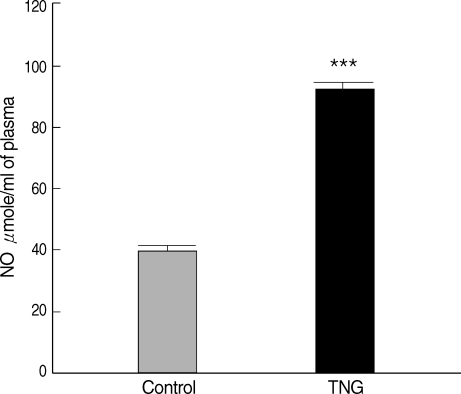
Fig. 2NO production in the liver and spleen suspensions of the control and TNG-treated BALB/c mice. NO levels in the suspensions of the liver and spleen of control (normal saline) and TNG groups of L. major infected BALB/c mice were assayed by Griess Microassay at the end of the experimental period. Significance of differences (*P < 0.05) was determined by an unpaired Student's t-test using Graph Pad Prism (n = 10 mice per group).
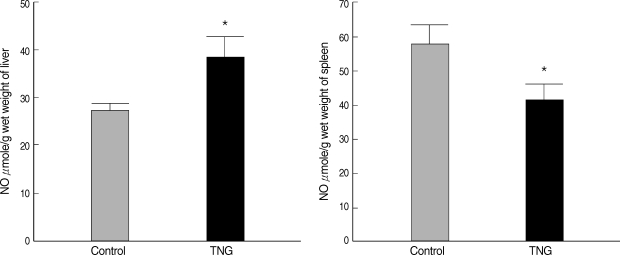
Fig. 3Progress of lesion sizes of CL in control and TNG-treated BALB/c mice. The lesion sizes of control (normal saline) and TNG groups of L. major infected BALB/c mice were measured in mm by a digital caliper in 2 diameters (D and d) at right angles to each other, and the size was determined according to the formula: S = (D + d) divided by 2. Significance of differences (*P < 0.05) was determined by one-way analysis of variances (ANOVA) and Student's t-test using Graph Pad Prism (n = 10 mice per group).
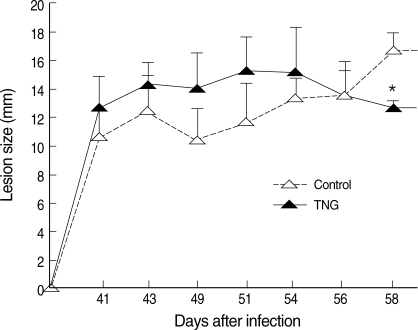
Fig. 4Percentages of positive Giemsa-stained smears from lesions and tissues of control and TNG-treated BALB/c mice. Giemsa-stained smears of control (normal saline) and TNG groups of L. major infected BALB/c mice were prepared from the lesion, liver, spleen, and lymph nodes at the end of the experimental period. Lesions were punctured at the margins and exudation material was smeared. Impression smears were prepared from organs as described in the methods for examination of amastigotes (leishman bodies) as an index of visceralization (n = 10 mice per group).
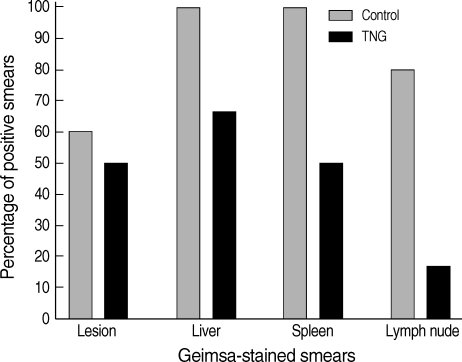
Fig. 5Comparative proliferation of amastigotes inside macrophages from cutaneous lesions of control and TNG-treated BALB/c mice. Proliferation of amastigotes inside macrophages was made by observation of Giemsa-stained smears of cutaneous lesions during the experimental period. The proliferation of parasites was evaluated by counting and calculation of mean numbers of amastigotes inside 5 random macrophages on Giemsa-stained lesion smears. Analysis of differences (**P < 0.01) was determined by an unpaired Student's t-test using Graph Pad Prism.
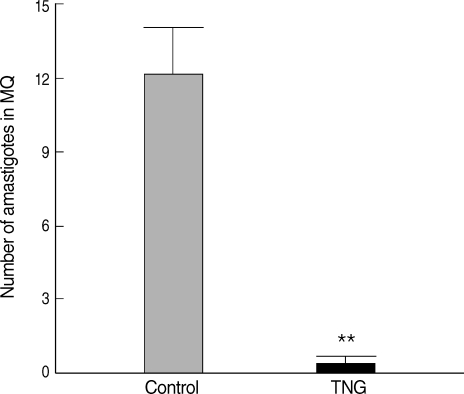
Fig. 6Pathophysiological evaluation of control and TNG-treated BALB/c mice. Pathophysiological signs, including hepatomegaly, splenomegaly (at the end of the experimental period), and body weight, were evaluated in 2 groups of L. major infected BALB/c mice with or without treatment with TNG. For measurement of hepatosplenomegaly, entire livers, and spleens were removed post-mortem from mice after terminal general anesthesia. The organ wet weights were measured as indices of possible hepatomegaly and splenomegaly. Body weight was measured using a top pan balance at different time of experiment. Significance of differences (*P < 0.05) was determined by an unpaired Student's t-test using Graph Pad Prism (n = 10 mice/groups).
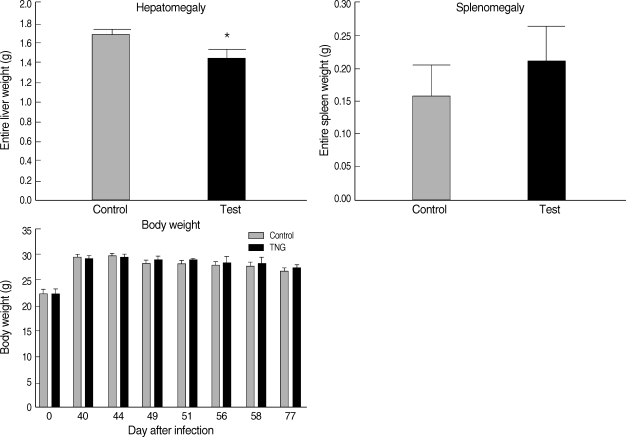
Fig. 7Survival rate of control and TNG-treated BALB/c mice. The survival rate was evaluated in 2 groups of L. major infected BALB/c mice with or without treatment with TNG at the end of the experimental period. The survival rate was presented as the percentage of survived experimental mice at different weeks after infection. Statistical analysis was applied by an unpaired Student's t-test using Graph Pad Prism (n = 10 mice/groups).