Abstract
The prevalence of the cercarial stage of an intestinal trematode, Haplorchis taichui, in thiarid snails (Gastropoda: Thiaridae) was investigated using light microscope and species-specific PCR procedures. A total of 988 snails were collected from Mae Taeng district, Chiang Mai province, northern Thailand, which comprised of 3 species; Melanoides tuberculata, Tarebia granifera, and Thiara scabra. The overall prevalence of pleurolophocercous cercariae was 21.7% as determined by the morphology. For genetic detection of H. taichui infection in snails, 2 primers Hapt_F (5'-GGCCAACGCAATCGTCATCC-3') and Hapt_R (5'-GCGTCGGGTTTCAGACATGG-3'), were used. The genomic DNA of H. taichui, which was used as a positive control, gave an amplification of the 256 bp fragment. The overall prevalence of H. taichui from specific PCR was 9.7%. The proportion of H. taichui among the pleurolophocercous cercariae in this study was 44.9%.
-
Key words: Haplorchis taichui, pleurolophocercous cercaria, thiarid snail, PCR, Thailand
The intestinal trematode,
Haplorchis taichui, is a medically important parasite infecting humans and livestock. Haplorchiasis has become one of the major public health threats in Asia and in parts of Africa and the Americas [
1-
3].
H. taichui is the most frequently reported species among the minute intestinal flukes from eastern Asia, including Thailand [
4-
7]. This parasite has an aquatic life cycle, using freshwater snails as the first [
8] and cyprinid fish as the second intermediate hosts, with definitive hosts being fish-eating mammals [
9,
10]. Previous reports showed that metacercariae of 2 heterophyids,
H. taichui and
Haplorchoides sp., had high prevalence of infection in cyprinoid fish collected from Chiang Mai and Lamphun provinces of northern Thailand [
11]. This was also found in a later study where a high prevalence (83.9%) of these 2 heterophyid trematodes was also recorded from the same area [
10].
Many species of heterophyid trematodes have been reported from pleurolophocercous cercariae, such as
H. taichui,
Haplorchis pumilio, and
Stellantchasmus falcatus [
12-
14]. About 100 species of snails have been reported to act as intermediate hosts for the trematodes especially in thiarid snails which harbor the larvae of intestinal trematodes, including
H. taichui [
12,
15].
Traditional methods of detecting cercarial infections are usually performed by exposing the snails to light or dissection. It is difficult to determine the parasite stages to the species level since they appear morphologically very similar to other trematodes [
16]. In addition, only a very small percentage of snails can be found to be infected when testing shed cercariae [
17]. Specific identification of cercariae from field collected snails is difficult, but necessary for epidemiological studies.
The development of a molecular approach for cercarial detection in infected snails is necessary and will be useful, such as PCR. Various conventional PCR assays have been developed to detect
H. taichui DNA in feces, definitive hosts, and intermediate hosts. Van et al. [
18] separated larvae and adult stages of
H. taichui and
H. pumilio using ITS-2 gene sequencing. Wongsawad et al. [
6] established the specific primer of
H. taichui by generating the 256 bp amplicon which had shown a positive result. The objectives of our study were to determine the prevalence of
H. taichui infections in snails by using PCR to gain information on the epidemiology of parasitic infections, and developing effective control measures.
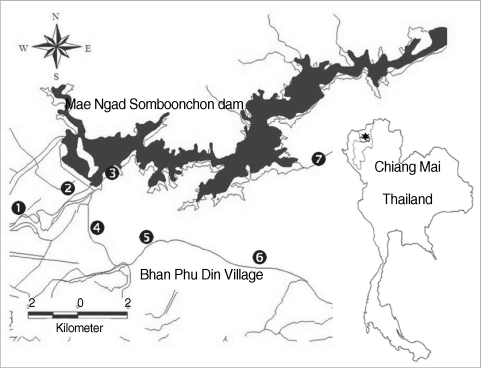
Field collections were made during March 2009 to February 2010. Thirty individuals of each thiarid snails were collected by handpicking and scoop methods. Seven sites were selected based on the relevance of public health concerns in and around the Mae Ngad Somboonchon dam in Mae Taeng district, Chiang Mai province, such as rice paddy, irrigation canal, dam, and river (
Fig. 1) during each of 3 seasons (hot-dry, rainy, and cool-dry). The snail specimens were identified using Brandt's taxonomic key [
19] and were examined for cercarial infections by crushing. The types of cercariae were morphologically identified under a compound microscope, and only pleurolophocercous cercariae were preserved in 70% alcohol for DNA extraction.
The genomic DNA was extracted and purified from individual pleurolophocercous cercariae samples found in each snail using commercial GF-1 tissue DNA extraction kit (Vivantis, Malaysia) according to the manufacturer's instructions. The genomic DNA of each sample was eluded in 50 µl of the elution buffer provided with the kit and stored at -20℃.
The
H. taichui specific primers were tested for specificity prior to perform the detection of cercarial stage by attempting to amplify them with all 6 adult trematode DNAs, including
H. taichui,
H. pumilio,
S. falcatus,
Centrocestus caninus,
Haplorchoides sp. and
Opisthorchis viverrini. These mentioned worms are widely distributed with a high prevalence of infection in Chiang Mai province, Thailand as same as they have been reported frequently in a past decade [
4,
5].
PCR amplification for the test of specificity and detection of cercarial stages of
H. taichui were also conducted using
H. taichui specific primers. There were 5'-GGCCAACGCAATCGTCATCC-3' (Hapt_F) as a forward primer and 5'-GCGTCGGGTTTCAGACATGG-3' as a reverse primer [
6]. The PCR amplifications were carried out in a final volume of 20 µl, including 1 µl of DNA template of pleurolophocercous cercariae from each snail sample, 0.6 µl of each primer (Hpt_F and Hpt_R), 0.3 µl of MgCl
2, dNTPs, and
Taq DNA polymerase. The amplification procedure involved an initial denaturation step at 95℃ for 5 min, then 35 cycle including denaturation at 95℃ for 45 sec, primer annealing at 68℃ for 45 sec, extension at 72℃ for 1 min, and final extension at 72℃ for 7 min. PCR products were analyzed after electrophoresis separation at 50 volt for 45 min on 2.5% agarose gels stained with ethidium bromide in TBE buffer. Gels were visualized by a Kodak Digital Camera (Gel Logic 100).
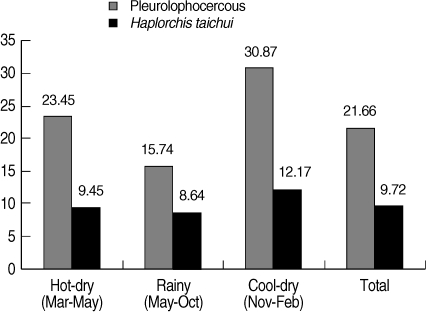
A total of 988 collected snails were identified to be 3 species, including
Melanoides tuberculata,
Tarebia granifera, and
Thiara scabra. Pleurolophocercous cercariae were found in 214 snails, with an overall prevalence of 21.7%, as detected by the morphology. The highest prevalence of infection was found in the cool-dry season (November - February) (30.87%), followed by hot-dry season (March - May) (23.45%), and rainy season (May - October) (15.74%) (
Fig. 2).
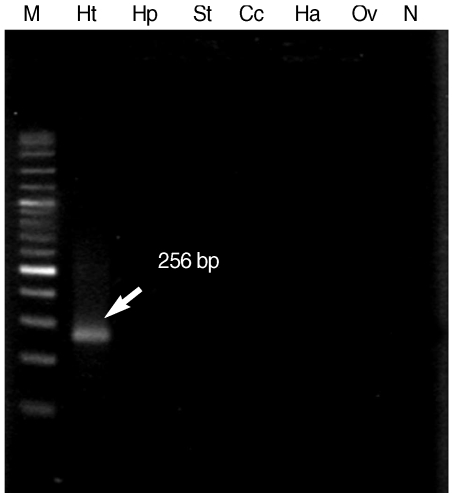
When
H. taichui specific primers were applied in combination to DNA from all 6 parasites, only 256 bp specific fragment of
H. taichui was generated and there was no cross reaction with any other tested trematodes (
Fig. 3). This result confirmed the specificity of
H. taichui specific primers which were appropriate for detection of cercariae in snails.
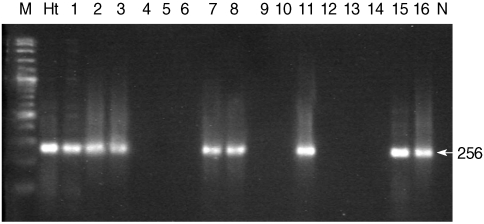
For the detection of
H. taichui in snails, 256 bp fragments were generated in different pleurolophocercous cercariae (
Fig. 4). Genomic DNA of
H. taichui adult worm was used as the positive control, and also gave an amplification product of 256 bp. This result provided the effective evidence by revealing that not only
H. taichui that was developed from pleurolophocercous cercariae, but also there were other trematode species which were generated from the same cercarial type, like
H. taichui. The overall prevalence of
H. taichui infected snails was 9.7% (
Fig. 2). The cool-dry season had the highest prevalence of 12.2%, followed by the hot-dry (9.5%) and rainy seasons (8.6%), respectively. The proportion of
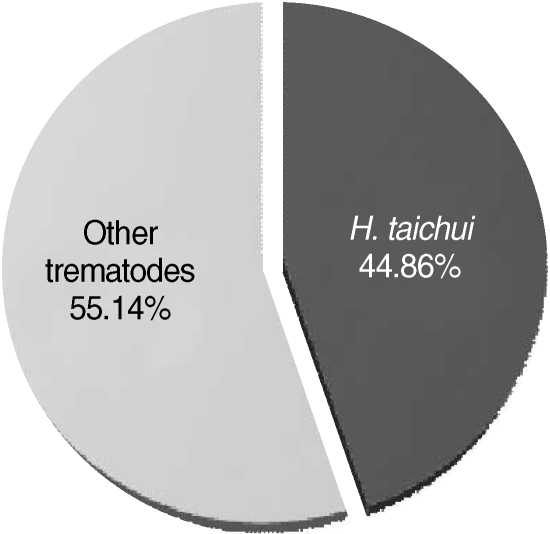
H. taichui among the pleurolophocercous cercarial population was 44.9% (96/214) (
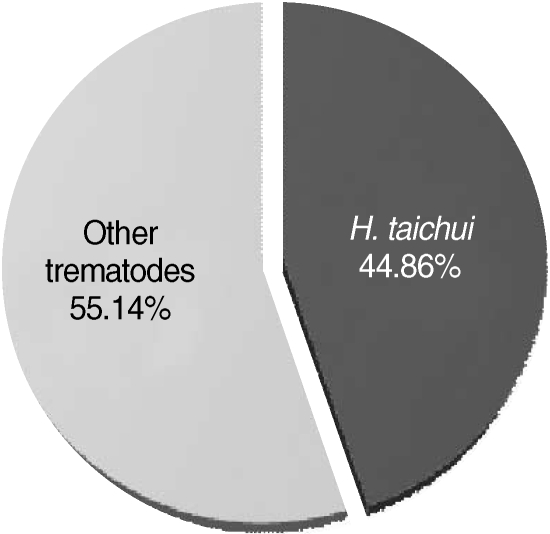
Fig. 5).
The prevalence of pleurolophocercous cercariae in naturally infected
M. tuberculata,
T. granifera, and
T. scabra in this study was relatively high (21.7%), and higher than in the previous study (5.3%) because the crushing method which was employed in this study show more sensitivity and accuracy than those of shedding method that acquired previously [
13]. Kumchoo et al. [
4] reported high prevalence (91.4%) of
H. taichui in cyprinoid fish from Mae Taeng district of Chiang Mai province. Nithikathkul and Wongsawad found a high infection rate of
H. taichui and
Haplorchoides sp. (83.90%) in this area [
10].
Our results showed that a high level (44.9%) of pleurolophocercous cercarial populations infected in thiarid snails was
H. taichui, whereas it can be proposed that the remainders can develop into other trematode species, such as
H. pumilio,
S. falcatus, and
Procerovum varium [
12-
14,
19,
20]. From this, it seems that the prevalence of trematode infection in definitive and second intermediate hosts collected from these areas may be very high. This conclusion is also supported by the peculiar ecological conditions of water resources in Mae Taeng district, which represents a complex and diverse freshwater ecosystem. There are good conditions for the cercarial stage of
H. taichui to develop in the molluscs and then fish which lead to perpetuation in their life cycle.
Freshwater snails are important for medical and veterinary public health points of view. About 100 species of snails have been reported to act as intermediate hosts, such as Thiaridae, and have been recorded to harbor larval trematodes [
22]. Our study found that
M. tuberculata and
T. granifera serve as intermediate hosts of
H. taichui. The PCR approach conducted in this study confirmed the high prevalence of
H. taichui, comprising almost a half of the pleurolophocercous cercariae. This test is suitable for epidemiological studies, and control programs for the snail intermediate hosts, and also for the detection of human infections. An essential and immediate application of this PCR method should be a metacercarial survey in fish of natural water resources in order to monitor infected areas. This may be a way for the long-term control of haplorchiasis in northern of Thailand.
ACKNOWLEDGEMENTS
We greatly appreciate use of the Applied Parasitology Research Laboratory and Economic Plants Genome Research and Service Center, Department of Biology, Faculty of Science, Chiang Mai University, Thailand. Special thanks are given to Mr. Sukson Chuboon and Mr. Tanu Marayong for their assistance in collecting specimens. We also thank Prof. Duangduen Krailas and Ms. Wivitcuta Dechruksa for confirming the identification of snail specimens. We thank Dr. J.F. Maxwell for editing our manuscript.
References
- 1. Chai JY, Murrell KD, Lambery AJ. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol 2005;35:1233-1254.
- 2. Dung DT, De NV, Waikagul J, Dalsgaard A, Chai JY, Sohn WM, Murrell KD. Fishborne zoonotic intestinal trematodes, Vietnam. Emerg Infect Dis 2007;13:1828-1833.
- 3. Pearson JC. A revision of the Subfamily Haplorchinae Looss, 1899 (Trematoda: Heterophyidae). Parasitology 1964;54:601-676.
- 4. Kumchoo K, Wongsawad C, Chai JY, Vanittanakom P, Rojanapaibul A. High prevalence of Haplorchis taichui metacercariae in cyprinoid fish from Chiang Mai province, Thailand. Southeast Asian J Trop Med Public Health 2005;36:451-455.
- 5. Thaenkham U, Visetssuk K, Dung DT, Waikagul J. Discrimination of Opisthorchis viverrini from Haplorchis taichui using COI sequence marker. Acta Trop 2007;103:26-32.
- 6. Wongsawad C, Wongsawad P, Chai JY, Anuntalabhochai S. Haplorchis taichui Witenberg, 1930: development of a HAT-RAPD marker for the detection of minute intestinal fluke infection. Exp Parasitol 2009;123:158-161.
- 7. Wongsawad C, Wongsawad P, Chuboon S, Anuntalabhochai S. Copro-diagnosis of Haplorchis taichui infection using sedimentation and PCR-based methods. Southeast Asian J Trop Med Public Health 2009;40:924-928.
- 8. Faltýnková A. Larval trematodes (Digenea) in molluscs from small water bodies near Ceske Budejovice, Czech Republic. Acta Parasitol 2005;50:49-55.
- 9. Dzikowski R, Levy MG, Poore MF, Flowers JR, Paperna I. Use of rDNA polymorphism for identification of Heterophyidae infecting freshwater fishes. Dis Aquat Organ 2004;59:35-41.
- 10. Nithikathkul C, Wongsawad C. Prevalence of Haplorchis taichui and Haplorchoides sp. metacercariae in freshwater fish from water reservoirs, 10. Chiang Mai, Thailand. Korean J Parasitol 2008;46:109-112.
- 11. Namue C, Rojanapaibul A, Wongsawad C. Occurrence of two heterophyid metacercariae Haplorchis and Haplorchoides in cyprinoid fish of some districts in Chiang Mai and Lumphun province. Southeast Asian J Trop Med Public Health 1998;29:401-405.
- 12. Dechruksa W, Krailas D, Ukong S, Inkapatanakul W, Koonchornboon T. Trematode infections of the freshwater snail Family Thiaridae in the Khek river, Thailand. Southeast Asian J Trop Med Public Health 2007;38:1016-1028.
- 13. Ukong S, Krailas D, Dangprasert T, Channgarm P. Studies on the morphology of cercariae obtained from freshwater snails at Erawan Waterfall, Erawan National Park, Thailand. Southeast Asian J Trop Med Public Health 2007;38:302-312.
- 14. Chuboon S, Wongsawad C. Molecular identification of larval trematodes in intermediate hosts from Chiang Mai, Thailand. Southeast Asian J Trop Med Public Health 2009;40:1216-1220.
- 15. Caron Y, Rondelaud D, Losson B. The detection and quantification of a digenean infection in the snail host with special emphasis on Fasciola sp. Parasitol Res 2008;103:735-744.
- 16. Pearson JC, Ow-Yang CK. New species of Haplorchis from Southeast Asia, together with key to the Haplorchis-group of Heterophyid trematode of the region. Southeast Asian J Trop Med Public Health 1982;13:35-60.
- 17. Haas W, Granzer M, Brockelman CR. Opisthorchis viverrini: finding the recognition of the fish host by the cercariae. Exp Parasitol 1990;71:422-431.
- 18. Van KV, Dalsgaard A, Blair D, Le TH. Haplorchis pumilio and H. taichui in Vietnam discriminated using ITS-2 DNA sequence data from adult and larvae. Exp Parasitol 2009;123:146-151.
- 19. Brandt RAM. The Non-Marine Aquatic Mollusca of Thailand. 1974, 1st ed. Germany. Arch Moll Band; pp 162-189.
- 20. Skov J, Kania PW, Dalsgaard A, Jørgensen TR, Buchmann K. Life cycle stages of heterophyid trematode in Vietnamese freshwater fishes traced by molecular and morphometric methods. Vet Parasitol 2009;160:66-75.
- 21. Umadevi K, Madhavi R. 2000. Observations on the morphology and life-cycle of Procerovum varium (Onji & Nishio, 1916) (Trematode: Heterophyidae). Syst Parasitol 2000;46:215-225.
- 22. Supian Z, Ikhwanuddin AM. Population dynamics of freshwater molluscs (Gastropoda: Melanoides tuberculata) in Crocker Range Park, Sabah. ARBEC 2002;1:1-9.
Fig. 1Map showing the 7 collecting sites in Mae Taeng district (★), Chiang Mai province, Thailand. 1. Rice paddy (N21"16.486'E0.4"09.359'), 2. River (N21"17.380'E0.50"02.067'), 3. Mae Ngad Somboonchon dam (N21"19.725'E0.50"03.645'), 4. Rice paddy (N21"13.134'E0.50"07.982'), 5. Irrigation canal (N21"12.864'E0.50"08.488'), 6. Stream (N21"12.992'E0.50"07.862'), and 7. Khek river (N19"08.775'E 0.99"00.586').
Fig. 2Seasonal prevalence of pleurophocercous cercariae using morphology, and H. taichui cercariae using PCR in the infected thiarid snails, collected in Mae Taeng district, Chiang Mai province of northern Thailand during March 2009 to February 2010.
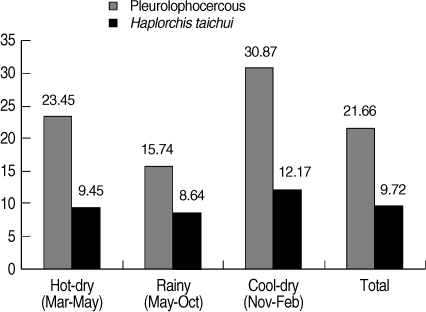
Fig. 3The 256 bp specific fragment (arrow) demonstrated the specificity of Hap_F and Hap_R specific primers, as confirmed by the positive result only in H. taichui DNA sample. Lane M, 100 bp molecular size markers; Lane Ht, H. taichui; Lane Hp, H. pumilio; Lane St, S. falcatus; Lane Cc, Centrocestus caninus; Lane Ha, Haplorchoides sp.; Lane Ov, Opisthorchis viverrini; Lane N, negative control.
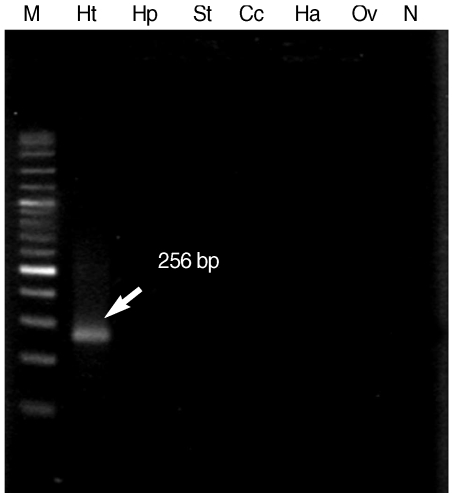
Fig. 4Representative agarose gel PCR profiles of Haplorchis taichui isolated from thairid snails. Lane M, molecular size maker; Lane Ht, H. taichui; Lanes 1-16, DNA samples of pleurolophocercous cercariae infected in snails (Lanes 1-3, 7, 8, 11, 15,16; positive results of H. taichui PCR specific fragment), (Lanes 4-6, 9,10, 12-14: negative results of cercariae infected in snails); Lane N, negative control.
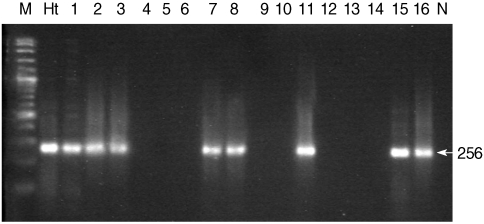
Fig. 5Proportion of Haplorchis taichui cercariae infected in thiarid snails (n = 214), as determined by PCR.