Abstract
A total of 286 individuals from 3 selected communities (Areedi-Aje, Ipakodo/Ojokodo, and Ijebu-Igbo) of Ijebu-North, southwestern Nigeria were examined for Loa loa microfilaremia using finger prick blood smear, between December 2008 and March 2009. Rapid assessment procedure for loiasis (RAPLOA) was used to obtain information, from 187 Ijebu-Igbo residents, on adverse reactions experienced from retrospective treatments with ivermectin and history of eye worm. Only 33.9% of the respondents reported having had a history of eye worm while 33.2% had microfilaremia. The demographic factor of gender was not significant determinants of the prevalence (P>0.05) while age was significant (P<0.05). The highest prevalence of eye worm history and microfilaremia were recorded in 61-70 and 15-20 years of age categories, respectively. Ijebu-Igbo had 27.3% eye worm history, 32.1% microfilaremia, and the highest intensity of 140 microfilariae (mf)/ml. Ipakodo area had the highest eye worm history of 54.4% and the highest intensity of 420 mf/ml. Areedi-Aje had the highest occurrence of 45.2% microfilaremia and the highest intensity of 460 mf/ml. Predictably, Areedi-Aje and Ipakodo areas were high risk communities. The low intensity of L. loa infection with an insignificant (2.1%; P>0.05) adverse reactions from 187 subjects involved in the retrospective ivermectin administration confirmed that ivermectin delivery may be considered safe. The community-directed treatment with ivermectin (CDTI) programme was most probably responsible for the low prevalence and intensity.
-
Key words: Loa loa, microfilaremia, ivermectin, adverse reaction, Nigeria
INTRODUCTION
About 1.2 million Nigerians are reported blind while 4.25 million adults aged 40 years and over have moderate to severe impairment or blindness [
1,
2]. River blindness is one of the common causes of blindness and has been a scourge for many years in the developing world, particularly in Sub-Saharan Africa. However, tremendous success has been achieved in combating against river blindness following the discovery and development of ivermectin (trade name Mectizan®) in 1987. Mectizan® has a major drawback, according to the Mectizan® Expert committee/The Mectizan® Donation Program and World Health Organization (WHO) [
3,
4], those suffering from onchocerciasis who simultaneously possess a high intensity of
Loa loa infection could potentially suffer from severe adverse effects (SAEs), including neurological reactions, such as encephalopathy. Several studies reported distribution of
L. loa and prevalences of human loiasis in the vegetational zones of Southwestern Nigeria [
5-
11]. Levels of adverse outcomes of treatment with ivermectin have been reported from areas of co-endemicity of onchocerciasis with loiasis or lymphatic filariasis [
12-
17]. Yet ivermectin remains the microfilaricide of choice in community-directed interventions. The Mectizan Donation Program commended the effectiveness and safe distribution of ivermectin [
3]. This is not without some kind of limitations which thus led to emphasis on increasing the level of awareness among the target communities for onchocerciasis and lymphatic filariasis treatment with ivermectin.
Enhancing the success story of African Programme for Onchocerciasis Control (APOC), it was further recommended that the prevalence of
L. loa and the risk of SAEs be assessed during or before commencement of ivermectin distribution on a community basis (
www.mectizan.org/loarecs.asp). Ijebu-North area of southwestern Nigeria has been reached by the annual ivermectin treatments. Hence, the need for assessing the risk status of loiasis endemicity and SAEs to the mass treatment with ivermectin program of Ijebu-North area is increasing. The objective of this study was to determine the current prevalence and intensity of loiasis in 3 communities in Ijebu-North as well as to investigate the suitability of community-directed treatment with ivermectin (CDTI) and the risk of SAEs in the study area.
MATERIALS AND METHODS
Study area and population
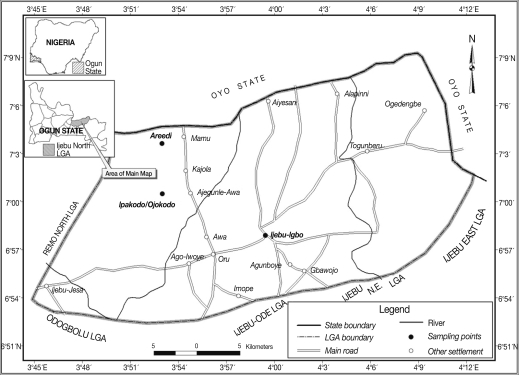
Ijebu-North area of southwestern Nigeria lies between latitude (Lat) 6.2° and 7.8° N and longitude (Long) 3.0° and 5.0°, within the rain forest vegetational zone. The climatic condition is a tropical pattern with rainy season starting in March and ending in November followed by a dry season. The mean annual rainfall varies from 128 cm in the southern part of the area to 105 cm in the northern area. Average monthly temperature ranges from 23℃ to 32℃. Ijebu-North area covers an area of about 1,250 km
2 and has its headquarters at Ijebu-Igbo. The people are mainly Ijebus (Yorubas). The study involved 3 communities; Ijebu-Igbo, Areedi-Aje, and Ipakodo/Ojokodo environs, which are 10, 17, and 13 km, respectively from Ago-Iwoye (Lat N 6° 56' 46.92'', Long E 3° 55' 55.09''), the state university town (
Fig. 1). Human population estimate at the time of the study was approximately 1,000, 500, and 1,000, respectively. The Ijebu-Igbo study group was primarily students, lecturers, technical, and administrative workers, while the inhabitants of the 2 villages were primarily subsistent farmers with few traders at Ipakodo.
The Ijebu-North local government area had annual mass ivermectin administration against onchocerciasis, at the time of observation, Ijebu-Igbo was reached with a second round of oral treatment, while Areedi-Aje and Ipakodo areas were reached for the first time. The health workers in charge of ivermectin distribution administered an optimal dose of 150 µg/kg body weight (that is, 2, 3 or 4 tablets depending on individual weight) were administered leaving out children aged less than 5 years or who weighed less than 15 kg or less than 95 cm tall, nursing mothers, menstruating women, and persons with severe illness or nervous disorders.
Advocacy and ethical clearance
Preliminary visits were made to the administrative headquarters of Ijebu-North local government area and approval was granted for the study protocol prior to commencement of the study. The local government health officer on ivermectin distribution and the representative from UNICEF assisted throughout the study. The purpose and methods of the study was discussed at length with the different community heads and head of school, who all gave their informed consent before the individual villagers were approached. The school community at Ijebu-Igbo was assembled by the school authority, for proper information dissemination. Only individuals who consented to participate were mobilized at the subsequent visits. Local knowledge about eye worm was recorded by administration of community level questionnaire to key informants (village heads/elders). Individuals who were 15 years and above, who had been resident in the localities for at least 5 years were informed of the objective of the study and their right to refuse to participate.
Study design and rapid assessment for loiasis (questionnaire)
A randomized cross-sectional survey was carried out between December 2008 and March 2009. A total of 286 participants were enrolled for the study; 187, 57, and 42 from Ijebu-Igbo, Ipakodo area, and Areedi-Aje, respectively. Individual questionnaire, designed according to rapid assessment procedure for loiasis (RAPLOA) guidelines [
18], to elicit responses to establish the history of eye worm and duration of eye worm episode (between 1-7 days) were administered with the help of an interpreter. The year of treatments with ivermectin during previous drug administration programme, adverse reaction, type, and duration of reaction following, if any, was recorded for individuals interviewed.
A 0.08 ml sample of finger-prick blood was collected on a clean slide, from each study participant between 11:00 and 15:00 hr. The use of 1 sterile blood lancet per participant was ensured, and used lancets were disinfected and properly disposed. Thick blood smears were made on the slide using standard procedures for Leishman's stain [
19]. The slides were examined microscopically with ×10 objective. Identification of
L. loa microfilariae was according to standard guidelines for diagnosis of microfilaremia [
20]. Microfilariae were counted and intensities were expressed as the number of microfilariae per ml blood.
Data were entered in summary of survey results form. Statistical Package for Social Science (SPSS) (version 10 package) was used to determine the relationship of rapid assessment of
L. loa indices with occurrence of adverse reactions following treatment with ivermectin. Percentage of people who had confirmed eye worm experience was determined for each locality. Classification of the communities as 'high risk' (>40% report of eye worm history) or 'low risk' (<40% report of eye worm history) was according to recommended classification [
21].
RESULTS
Preliminary observations
Local name for the eye worm in the study sites was "aran oju" meaning worm of the eye. A 80% of the interviewees already had awareness of loiasis.
Prevalence of loiasis
A 33.9% of respondents reported history of
Loa eye worm infection, while 33.2% had
Loa microfilaremia; these 2 prevalences were not significantly different (
P>0.05). Ipakodo/Ojokodo had the highest prevalence of eye worm history, 54.4%, while Ijebu-Igbo had the least, 27.3%. The prevalence of microfilaremia was the highest in Areedi-Aje, followed by Ijebu-Igbo and the least was Ipakodo area (
Table 1). Females had a high prevalence of
Loa infection. However, there were no significant differences (
P>0.05) in the history of eye worm infection and microfilaremia in relation to gender (
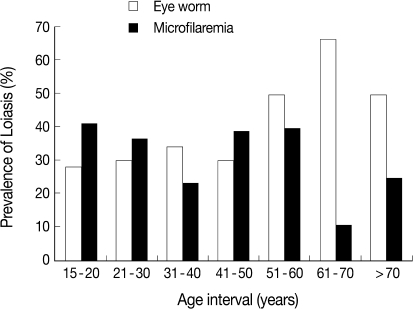
Table 2). The age category of 61-70 years had the highest prevalence of eye worm history (66.7%) and the lowest prevalence of microfilaremia (11.1%), while the age category of 15-20 years recorded the lowest prevalence of eye worm history (28.3%) and the highest prevalence of 41.3%
loa microfilaremia (
Fig. 2). The difference between prevalences in age groups 15-20 and 61-70 years was statistically significant (
P<0.05).
Loa microfilarial (mf) intensity among interviewees ranged from 20-460 mf/ml. At the community level, mf intensity ranges were 60-460 mf/ml, 80-420 mf/ml, and 20-140mf/ml at Areedi-Aje, Ipakodo/Ojokodo, and Ijebu-Igbo, respectively (
Table 1).
Adverse reactions following retrospective treatment with ivermectin
Adverse outcomes were reported in only 4 participants (
Table 3). Chi-square test showed insignificant differences between males and females (
P>0.05). Adverse events reported were itching on the head for 1 day in 1 patient, dizziness for 2 days in another, stomach ache for 2 days in 1 subject, and worm movement along the white part of the eyes for 1 month in yet another. Pre-treatment densities of microfilariae for the 2.1% reactors are shown in
Table 3. The nature of adverse events was mild, and no case of severe adverse reaction, involving the nervous system, was reported.
DISCUSSION
Ivermectin remains the prominent safe and efficacious microfilaricide but because it has little effect on the adult worm, retreatment of individuals annually is recommended [
22]. The recent past years witnessed continuous administration of ivermectin in Ijebu-North of southwestern Nigeria as treatment for either onchocerciasis or lymphatic filariasis or both. All 3 surveyed communities, already reached by masstreatment with ivermectin, showed a high level (80%) awareness of eye worm infection. The same local name for
L. loa eye worm was available, which further showed there is widespread knowledge of the
Loa eye worm.
There are reports of the long lasting effect of ivermectin on
L. loa microfilaremia [
13] and observations that a history of ivermectin treatment for longer than 3 years tended to decrease the probability that a subject had a detectable
L. loa mf [
23]. These factors could explain why prevalence of 33.9% history of eye worm (restricted definition) was observed in the present study. This prevalence falls under the 'low risk' category because a prevalence of more than 40% eye worm history is the threshold for a high risk of adverse reactions [
21,
24,
25].
The community related prevalence observed in the present study indicated that there is need for determination of the endemicity status of L. loa in individual community qualified for CDTI, before the commencement of drug administration. A significant variation ranging from 27.3%, 35.7%, to 54.4% prevalence of loiasis was observed at the communities. The implication is that while Ijebu-Igbo fell under 'low risk' of adverse reaction, Ipakodo and environs came under a 'high risk' area, all within the same zone with 33.9% prevalence. Thus, putting the usage of 40% cut off point for safe distribution of ivermectin in a bioecological zone at risk of false threshold above which there is a high risk of adverse reactions.
A linear relationship between the prevalence and intensity of
L. loa infection was reported in 102 surveyed villages [
25], which indicated a high intensity of infection (>8,000 mf/ml) above 5% which corresponded to a prevalence of microfilaremia in thick blood film of >20%. The prevalence of eye worm according to the restricted definition was reported to clearly increase with the prevalence of microfilaremia. The cut-off point of a 40% prevalence of eye worm corresponded to a 20% prevalence of microfilaremia at the community level. The instability of the population in the present study and exclusion of less than 5 years residents as expected, with a suspected possible neglected reservoir of
Loa parasites could account for why microfilaremia may be high or still be on the increase (33.2% prevalence against 33.9% prevalence of eye worm). However, the low intensity of infection ranging from 20-460 mf/ml showed impact of CDTI in the study area.
Loiasis distribution status is not significantly gender-biased, but it is significantly age-related. Female participants showed higher positive
Loa microfilaria than males, which were not significantly different (
P>0.05) and could only suggest that females were more exposed to
Chrysops vectors than males in the study area. On the contrary, earlier studies in Nigeria had reported that males were likely than females to present with
Loa microfilaremia [
11,
26,
27]. The mechanisms underlying the gender bias are still unknown; experimental infections in animal models have demonstrated gender differences in susceptibility to, or the development of, filarial infections [
28]. Epidemiological data from central Cameroon revealed that prevalence of
Loa microfilaremia was significantly higher in male subjects than in females [
23], the authors however concluded based on level of exposure (higher or low) that males and females do not have the same pattern of response to exposure and that gender and exposure were associated with the presence of
L. loa microfilaremia. Some other surveys had reported that males were more likely to present with
Loa microfilaremia than females [
23,
29].
Earlier workers [
30] did not find any significant association between gender and microfilaremic status; however, some observed lower prevalences of
Loa microfilaremia in females. A question of interest should be the peculiarity of the locality in terms of sociocultural attitudes and practices. The observations of the present study could only confirm the possibility of equal vector exposure both on the farmlands visited by males and females and temporary human settlements in the immediate vicinity of the vectors.
There is an inverse relationship between the age and body immunity of a man [
31]. The higher the age, the more susceptible to infections man becomes. The possibility of occupational hazard was also suggested as majority of the adults in the study were farmers and highly exposed to the vector transmitting the disease. The present study also confirmed that eye worm history was more prevalent in the age group of 61-70 years. In addition, the significant differences observed in the prevalence by age could be due to differences in vector exposure. An indication pointed out by earlier investigators [
23] reported indications that an effect of ivermectin may be to paralyze the
Loa mf. This according to the previous report may facilitate the action of inflammatory cells against the mf, which could then be destroyed in the blood vessels or in the lymphatic system. There was a confirmation of the previous report of long-term effect of ivermectin on reproductive activity, or longevity, of the adult worms [
32] which also indicated that ivermectin may also reduce the production of new mf [
23]. This could explain the present observation of least mf in the age group of 61-70 years who had been treated with ivermectin in the past. An expected increase in the prevalence of microfilaremia with age was reported by earlier workers [
23,
30]. A further suggestion explained that the risk of an individual being infected, via the bite of an infected
Chrysops, increases with time, and that a person who has become microfilaremic remains so throughout life. Continued drug administration had been reported to result in reduced intensity of adverse reactions, prevalence, and intensity of microfilariae [
33]. Furthermore, the probability of marked reduction of loiasis transmission by a single dose of ivermectin had been reported [
34]. The microfilaremic status of the subjects in the present study could be further explained by the reported observation that microfilariae are never detected in samples of peripheral blood from many people who are known to have been infected with
L. loa and have experienced occult loiasis [
35,
36]
ACKNOWLEDGEMENTS
We gratefully acknowledge the assistance and cooperation of Ijebu-North Local Government Health Division (the community Health Officer, Mrs. Awobona and the UNICEF Representative, Mr. Hamzat, Drs. Akinlolu and Akinbajo in particular) and Ogun State Ivermctin Distribution Unit.
References
- 1. Kyari F, Gudlavalleti MV, Sivsubramaniam S, Gilbert CE, Abdull MM, Entekume G, Foster A. Nigeria National Blindness and Visual Impairment Study Group. Prevalence of blindness and visual impairment in Nigeria: the National Blindness and Visual Impairment Study. Invest Ophthalmol Vis Sci 2009;50:2033-2039.
- 2. Ashaye AO. Glaucoma blindness: facts, fancies and fables. 12th Faculty of Ophthalmology Lecture, National Postgraduate Medical College of Nigeria. 2010, p 48.
- 3. The Mectizan® Expert Committee. The Mectizan®Donation Program. Recommendations for the treatment of onchocerciasis with Mectizan® in areas co-endemic for onchocerciasis and loiasis. 2004.
- 4. "A New Drug for River Blindness?". World Health Organization. 2007. www.who.int/tdr/ir/moxidectin.pdf
- 5. Ogunba EO. Loiasis in Ijebu division, West Nigeria. Trop Geogr Med 1971;23:194-200.
- 6. Ogunba EO. Ecology of human loiasis in Nigeria. Trans R Soc Trop Med Hyg 1972;66:743-748.
- 7. Oyerinde JP, Odugbemi T, Fagbenro-Beyioku AF. Investigation of filarial worms of man in metropolitan Lagos. Acta Trop 1988;45:191-192.
- 8. Agbolade OM, Akinboye DO. Loiasis and some haematological parameters in Ijebu-North area of Ogun State, Nigeria. Bull Sci Ass Nig 2000;23:29-32.
- 9. Agbolade OM, Akinboye DO. Loa loa and Mansonella perstans infections in Ijebu-North, Western Nigeria; a Parasitological Study. Jpn J Infect Dis 2001;54:108-110.
- 10. Agbolade OM, Akinboye DO, Ogunkolo OF. Loa loa and Mansonella perstans: neglected human infections that need control in Nigeria. Afr J Biotech 2005;4:1554-1558.
- 11. Adeoye GO, Akinsanya B, Otubanjo AO, Ibidapo CA, Afolabi T, Okwuzu I, Adejai EO, Braide EEI. Prevalence of loiasis in Ondo State, Nigeria, as assessed by the rapid assessment procedure for loiasis (RAPLOA). Ann Trop Med Parasitol 2008;102:215-227.
- 12. Chippaux JP, Boussinesq M, Gardon JN, Gardon-Wendel N, Ernould JC. Severe adverse reaction risk during mass treatment with ivermectin in loiasis- endemic areas. Parasitol Today 1996;12:448-450.
- 13. Gardon J, Gardon-Wendel N, Demanga-Ngangue , Kamgno J, Chippaux JP, Boussinesq M. Serious reactions after treatment of onchocerciasis with ivermectin in an endemic area for Loa loa infection. Lancet 1997;350:18-22.
- 14. Boussinesq M, Gardon J, Gardon-Wendel N, Kamgno P, Ngoumou P, Chippaux JP. Three probable causes of Loa loa encephalopathy following ivermectin treatment for onchocersiasis. Am J Trop Med Hyg 1998;58:461-469.
- 15. Kipp W, Bamhuhiiga J, Rubaale T, Buttner DW. Adverse reaction to ivermectin treatment in Simulium neavei - transmitted onchocersiasis. Am J Trop Med Hyg 2003;69:621-623.
- 16. Oyibo WA, Fagbenro-Beyioku AF. Adverse reaction following annual ivermectin treatment of onchocerciasis in Nigeria. Int J Infect Dis 2003;7:156-159.
- 17. World Health Organisation. UNDP/World Bank/WHO TDR/IDE/CDDI/D3.1. Report of a multi-country study: the involvement of community-directed distributors of ivermectin in other health and development activities. 2003.
- 18. World Health Organization. Doc TDR/IDE/RAPLOA/021. Guidelines for Rapid Assessment of Loa loa. 2002, Geneva. WHO.
- 19. Brown BA. Haematology: Principles and Procedures. 1980, 3rd ed. England. Lea and Febiger.
- 20. Orihel TC, Ash LR, Ramachandran CP, Ottensen EA. Bench Aids for the Diagnosis of Filarial Infections: Geneva World Health Organization. 1997.
- 21. Ranque S, Garcia A, Boussinesq M, Gardon NJ, Kamgno J, Chippaux JP. Decreased prevalence and intensity of Loa loa infection in a community treated with ivermectin every three months for two years. Trans R Soc Trop Med Hyg 1996;90:429-430.
- 22. Hodgkin C, Molyneux DH, Abiose A, Philippon B, Reich MR, Remme JH, Thylefors B, Traore M, Grepin K. The future of onchocerciasis control in Africa. PLOS Negl Trop Dis 2007;1:e74.
- 23. Pion SDS, Demanou B, Oudi N, Boussinesq M. Loiasis: the individual factors associated with the presence of microfilaremia. Ann Trop Med Parasitol 2005;99:491-500.
- 24. Tropical Diseases Research News. Keynote Article. Loa loa: a new rapid assessment tool. TDR News 2001;66:1-2.
- 25. Rapid Assessment Procedures for Loiasis. World Health Organization. 2001. www.who.int/tdr/cdpublicaions/pdf/raploa.pdf
- 26. Anosike JC, Onwuliri CO. Studies on filariasis in Bauchi State, Nigeria: the prevalence of human filariasis in Darazo local governmentarea. Appl Parasitol 1994;35:242-250.
- 27. Udonsi JK. Filariasis in Igwun River Basin, Nigeria: an epidemiological and clinical study with a note on the vectors. Ann Trop Med Parasitol 1988;82:75-82.
- 28. Ash LR. Preferential susceptibility of male jirds (Meriones unguiculatus) to infection with Brugia pahangi. J Parasitol 1971;57:777-780.
- 29. Pion SDS, Gardon NJ, Kamgno J, Gardon-Wendel N, Chippaux JP, Boussinesq M. Structure of the microfilarial reservoir of Loa loa in the human host and its implications for monitoring the programmes of community-directed treatment with ivermectin carried out in Africa. Parasitology 2004;129:613-629.
- 30. Garcia A, Abel L, Cot M, Ranque S, Richard P, Boussinesq M, Chippaux JP. Longitudinal survey of Loa loa filariasis in Southern Cameroon: long-term stability and factors influencing individual microfilarial status. Am J Trop Med Hyg 1995;52:370-375.
- 31. Pinder M, Duport A, Egwag TG. Identification of a surface antigen on Loa loa microfilaraemic state in man. J Immunol 1988;141:2480-2486.
- 32. Martin-Prevel Y, Cosnefroy J, Tshipamba P, Ngari P, Chodakewitz JA, Pinder M. Tolerance and efficacy of single high-dose ivermectin for the treatment of loiasis. Am J Trop Med Hyg 1993;48:186-192.
- 33. Burnham GM. Adverse reactions to ivermectin treatment for onchocerciasis: results of a placebo-controlled, double-blind trial in malaria. Trans R Soc Trop Med Hyg 1993;87:313-317.
- 34. Duong TH, Kombila M, Ferrer A, Bureau P, Gaxotte P, Richard-Lenoble D. Reduced Loa loa microfilaria count ten to twelve months after a single dose of ivermectin. Trans R Soc Trop Med Hyg 1997;91:592-593.
- 35. Pinder M. Loa loa-a neglected filaria. Parasitol Today 1988;4:279-284.
- 36. Wahl G, Georges AJ. Current knowledge on the epidemiology, diagnosis, immunology and treatment of loiasis. Trop Med Parasitol 1995;46:287-291.
Fig. 1Map of Ijebu-North local government area showing the study sites.
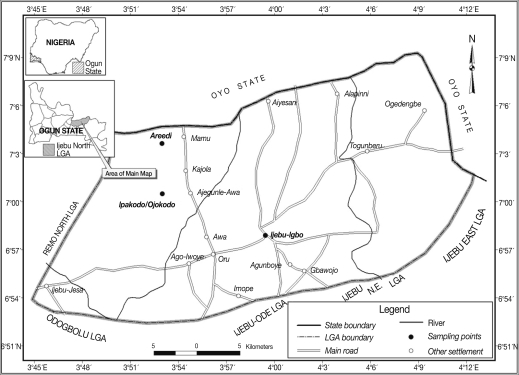
Fig. 2Prevalence of Loa eye worm history and Loa microfilaremia in relation to age group.
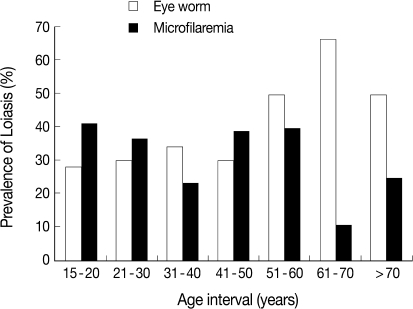
Table 1.Prevalence of loiasis in 3 communities of Ijebu-North area
Table 1.
|
Locality |
No. of residents examined |
History of eye worm Infection (%)
|
Blood examination (%)
|
Range of microfilarial density |
|
Yes |
No |
Yes |
No |
|
Areedi-Aje |
42 |
15 (35.7) |
27 (64.3) |
19 (45.2) |
23 (57.8) |
60 - 460 |
|
Ipakodo/Ojokodo |
57 |
31 (54.4) |
26 (45.6) |
16 (28.1) |
41 (71.9) |
80 - 420 |
|
Ijebu-Igbo |
187 |
51 (27.3) |
136 (72.7) |
60 (32.1) |
127 (67.9) |
20 - 140 |
|
Total |
286 |
97 (33.9) |
189 (66.1) |
95 (33.2) |
191 (66.8) |
|
Table 2.Gender-based prevalence of loiasis
Table 2.
|
Gender |
History of Loa eye worm; % (count)
|
Loa microfilaremia; % (count)
|
|
Yes |
No |
Positive |
Negative |
|
Female |
31.3 (50) |
68.8 (110) |
32.5 (52) |
67.5 (108) |
|
Male |
37.3 (47) |
62.7 (79) |
34.1 (43) |
65.9 (83) |
Table 3.Adverse consequences following ivermectin treatment
Table 3.
|
No. |
Gender |
Age |
Nature of adverse reaction |
Loa loa eye worm history |
Loa microfilarial density (mf/ml) |
|
1 |
Female |
41 |
Itching on the head for 1 day |
No |
80 |
|
2 |
Female |
22 |
Dizziness for 2 days |
Yes |
100 |
|
3 |
Male |
24 |
Stomach ache for 2 days |
No |
60 |
|
4 |
Male |
44 |
Worm moving along the white part of the eyes for more than 7 days |
Yes |
120 |