Abstract
In order to get a better understanding of the role of protease-activated receptor 2 (PAR2) in type 2 helper T (Th2) cell responses against Trichinella spiralis infection, we analyzed Th2 responses in T. spiralis-infected PAR2 knockout (KO) mice. The levels of the Th2 cell-secreted cytokines, IL-4, IL-5, and IL-13 were markedly reduced in the PAR2 KO mice as compared to the wild type mice following infection with T. spiralis. The serum levels of parasite-specific IgE increased significantly in the wild type mice as the result of T. spiralis infection, but this level was not significantly increased in PAR2 KO mice. The expression level of thymic stromal lymphopoietin, IL-25, and eotaxin gene (the genes were recently known as Th2 response initiators) of mouse intestinal epithelial cells were increased as the result of treatment with T. spiralis excretory-secretory proteins. However, the expression of these chemokine genes was inhibited by protease inhibitor treatments. In conclusion, PAR2 might involve in Th2 responses against T. spiralis infection.
-
Key words: Trichinella spiralis, protease-activated receptor 2, IL-25, thymic stromal lymphopoietin (TSLP)
INTRODUCTION
Despite their differing morphologies, physiologies, and life cycles, many parasitic helminths evoke similar immune responses in their hosts; the most notable response is a Th2 response characterized by an increase in IL-4 and IL-5 expression, high IgE level, and eosinophilia. The manner in which parasites elicit this common immune response in their host remains unclear. However, it should be possible to extend that common receptors exist in the host animals, which are able to detect similar molecules from several types of helminth parasites, such as the Toll-like receptors (TLRs) [
1,
2]. One of the most interesting features of helminthic parasites is that most of them harbor various types of proteases for host tissue invasion or digestion, or for molting over their life cycle. Until now, many proteases have been identified as virulence or pathogenic factors from helminthic parasites [
3]. When certain nematode larvae migrate in their host internal organs (lung migration, especially alveoli invasion), they evoke parasite-specific IgE-mediated hypersensitivity. At that time, some protease is required for tissue digestion. Additionally, some parasite proteases, such as DerP1, induce a strong allergic reaction in humans. The reason for this potent IgE eliciting property of the parasite is likely to be attributed to its protease activity [
4-
6].
We concentrated on some receptors that could be activated by proteases; the protease-activated receptors (PARs) is one of those. PARs belong to the recently described family of G protein-coupled, 7-transmembrane domain receptors [
7]. PARs are activated via the proteolytic cleavage of their N-terminal domain by proteinases, thereby resulting in the generation of a new N-terminal "tethered ligand", which can autoactivate the receptor function [
8]. Four members (PAR1-PAR4) of the PAR family have been cloned. PAR1, PAR3, and PAR4 can be activated by thrombin, and PAR2 can be activated by trypsin, mast cell tryptase, neutrophil protease 3, tissue factor/factor VIIa/factor Xa, membrane-tethered serine proteinase-1, or proteases from
Porphyromonas gingivalis [
9]. There have been a few studies conducted regarding the relationship between helminthic infections and PAR2. Devil et al. [
1] previously suggested that PAR2 is a recognition receptor for
Nippostrongylus brasiliensis proteases.
Nematodes of the genus
Trichinella infect a broad range of mammals, birds, and reptiles. These parasites alternate during their life cycles, between enteric stages and skeletal muscle stages, within their hosts.
T. spiralis larvae have been shown to be able to establish chronic infections in the skeletal muscles of immunocompetent hosts [
10]. They also expressed a greater variety of proteases, particularly serine protease and aspartic protease, in larval stages as compared to the adult worm stage, and these proteases might be necessary to infect new hosts [
11-
13].
T. spiralis could evoke a host Th2 immune response, and the host also elevates its Th2 immune responses in order to eliminate the worms [
14]. However, the mechanisms underlying the initiation of the Th2 host response against
T. spiralis infection have yet to be clearly elucidated.
In the present study, we analyzed the Th2 chemokine gene expression levels induced by T. spiralis infection or excretory-secretory (ES) protein stimulation, and attempted to determine whether T. spiralis infection could evoke Th2 response via PAR2 or not, using PAR2 knockout (KO) mice.
MATERIALS AND METHODS
Parasite
The strain of T. spiralis was maintained in our laboratory via serial passage in rats. To acquire infectious muscle larvae, eviscerated mouse carcasses were cut into pieces and then digested in 6% pepsin-hydrochloride digestion fluid (artificial gastric juice) overnight at 37℃ with stirring. The larvae were corrected manually from muscle digested solution under microscopy and washed 6 times with sterile PBS. After collection, in order to prevent any contamination with the host material, the worms were thoroughly and carefully washed several times over a 3-hr period in PBS.
Preparation of ES proteins and total extracts of muscle larvae
To obtain ES proteins, collected worms were introduced into sterile flasks with serum-free RPMI 1640 medium supplemented with antibiotics (100 µg/ml Pen/Strep; Gibco, Carlsbad, California, USA). The culture was then maintained for 7 consecutive days at 37℃ in 5% CO2. It was confirmed that, during this time, the majority of the larvae remained alive and evidenced good mobility. Following centrifugation (12,000 g for 30 min), the supernatants were concentrated by pressure applied in a concentrator (Amicon, Millipore Corporations, Billerica, Massachusetts, USA) with 3,000 Da pore size membranes. Various proteins (3-100 kDa and heavier) were detected in SDS-PAGE. The unnecessary excessive salts were eliminated from collected medium by HiTrap Desalting™ (GE Healthcare, Uppsala, Sweden) and dialyzed against PBS for 24 hr with continuous agitation in a cold room in order to eliminate any antibiotic remnants. Lipopolysacharide (LPS) was depleted (endotoxin levels < 0.01 µg/ml) from ES proteins using Detoxi-Gel Affinity Pak prepacked columns (Pierce, Rockford, Illinois, USA), in accordance with the manufacturer's instructions. To obtain total extracts of larval somatic proteins, the larvae were grinded using tissue homogenizer in sterile PBS. The total extract of T. spiralis larva was obtained using PRO-PREP protein extraction solution (Intron Biotechnology, Sungnam, Korea). The extraction procedures were carried out according to the manufacturer's recommended protocols. The total extract was also depleted of LPS like ES proteins.
Mice and experimental design
Female C57BL/6 mice at the age of 5 weeks were purchased from Samtako Co. (Gyeonggi-do, Korea). PAR2-/- mice (C57BL/6 background) were purchased from Jackson Laboratory Co. (Bar Harbor, Massachesetts, USA) and were bred in a SPF facility at the Institute for Laboratory Animals of Pusan National University, Busan, Korea. This study included 2 groups of mice, consisting of 3-5 mice per group. C57BL/6 wild type (WT) and PAR2 KO mice were orally infected with 250 infectious T. spiralis larvae, and were sacrificed after 4 weeks of infection. T. spiralis larvae were counted on straight muscles following a histological process for known degree of infection, and the sizes of the spleens were measured. To determine the incidence of specific cytokine secreting lymphocytes, we isolated lymphocytes from the spleen and mesenteric lymph nodes (MLNs) of mice infected with T. spiralis. The cytokine levels in the culture supernatants of lymphocytes and T. spiralis-specific immunoglobulin levels in serum were measured.
Analysis of cytokine-producing lymphocytes
After the mice were sacrificed, their spleen and MLNs were detached from mice. The spleen and MLNs were disrupted and treated with ACK hypotonic lysis solution (Sigma-Aldrich, St. Louis, Missouri, USA) for 2 min at room temperature for RBC lysis. After then, the remaining cells were filtered with 100 µm mesh (Small Parts, Inc. Seattle, Washington, USA), and the cells were plated in 48-well plates as 5×106 cells/ml in RPMI 1640 with 10% fetal bovine serum and penicillin/streptomycin. For the CD3 stimulation experiments, 0.5 µg/ml of CD3 antibody (eBioscience, San Diego, California, USA) was added to cell-plated wells. Plated cells were incubated for 72 hr at 37℃ in an atmosphere of 5% CO2. Following incubation, the culture media was harvested and stored at -20℃. ELISA tests to measure IL-4, IL-5, IL-13, and IFN-γ in culture supernatants with an ELISA kit (eBioscience). The assay was conducted in accordance with the manufacturer's recommended protocols. ELISA analysis was conducted for a total of 3 times.
Total and T. spiralis immunoglobulin analysis
After the mice were sacrificed, serum samples were collected from mice via cardiac puncture. Total and parasite-specific IgG1, IgG2a, and IgE levels were determined by ELISA. The 96-well immune plates (Nunc, Roskilde, Denmark) were coated at 37℃ until dry with 1 µg/ml of anti-mouse IgG1, IgG2a, and IgE, respectively, or 2.0 µg/ml of ES proteins and total extracts from T. spiralis in 0.1 M sodium carbonate buffer, at a pH of 9.6. After 3 washes with 0.1% Tween 20 contained PBS (Sigma), the serum samples were diluted (1:40-1:105 for IgG1 and IgG2a) in sample buffer (PBS supplemented with 0.1% Tween 20 and 2% BSA), then added to the plate and incubated for 2 hr at 37℃. After incubation, the plate was washed 3 times and anti-mouse antibodies (BD Bioscience, San Jose, California, USA) were added to the plate and incubated for 1 hr at 37℃. After washing, the plate was incubated for 30 min at room temperature with streptavidin-HRP (BD Bioscience). For color development, tetra-methyl-benzidine was utilized as a substrate. The reaction was halted with a half-volume of an H2SO4 solution (Merck, Darmstadt, Germany). The absorption was then measured at 450 nm.
Zymography
Gelatin-gel was prepared that the running gel add gelatin-stock solution (10 mg/ml in H2O) to get the gelatin concentration of 0.1% (1 mg/ml). The ES proteins, boiled ES protein, and protease inhibitor-pretreated ES protein were mixed with 2×Sample Buffer (1 M Tris pH 6.8, 1% bromphenol blue, SDS, glycerol, β-mercaptoethanol) and set at 10 min at room temperature, and the gel was run with 1×Tris-Glycine SDS Running Buffer (0.025 M Tris, 0.192 M Glycine, pH 8.5, 0.1% SDS) (125 V for 2 hr). After running, the gel was washed to remove the SDS and re-natured proteinase activity with Zymogram Renaturing Buffer (2.5% Triton X-100). The gel was developed with Zymogram Developing Buffer (0.05 M Tris-HCl ph 7.6, 0.2 M NaCl, 5 mM CaCl2, 0.2% Brij) during 30 min at room temperature. The buffer was then replaced with fresh 1xZymogram Developing Buffer and the gel was incubated at 37℃ for 4 hr. The gel was stained with Coomassie Blue R-250 for 30 min and destained with an appropriate Coomassie R-250 destaining solution.
Cell culture and in vitro stimulation
Mouse colon epithelial cell line, CT-26 cells, were maintained in Dulbecco's modified Eagle's medium (DMEM) (Hyclone, Road Logan, Utah, USA), supplemented with 10% heat-inactivated FBS (Hyclone), 100 units/ml penicillin, and 100 µg/ml streptomycin. Then 4×105 cells were plated in 24-well plates and incubated overnight at 37℃ containing 5% CO2. One µg of ES protein from T. spiralis was applied to cells for 2 hr and the total RNA was extracted.
RNA extraction and real-time PCR
After treatment, 4×10
5 CT-26 cells were collected in 1 ml of QIAzol (Qiagen science, Valencia, California, USA), and RNA extraction was conducted in accordance with the manufacturer's protocols, transcribing 2 µg of RNA using moloney murine leukemia virus (M-MLV) reverse transcriptase (Promega, Madison, Wisconsin, USA). The Eotaxin, TSLP, IL-25 RNA levels were determined by real-time PCR using iCycler™ (Bio-Rad Laboratories, Inc., Hercules, California, USA) real-time PCR machines. GAPDH was utilized as the reference gene. The primer sequences are provided in
Table 1. PCR amplification was conducted in a 20 µl volume, containing 2 µl cDNA, 0.2 µM primers, and the SYBR Premix
Ex Taq™ (TAKARA Bio Inc., Otsu, Shiga, Japan). All genes were amplified using the following conditions: 1 min 30 sec host start at 95℃, followed by denaturation at 95℃ for 25 sec, primer annealing at 55℃ for 20 sec, and elongation at 72℃ and 30 sec for 40 cycles. The fluorescent DNA-binding dye SYBR was monitored after each cycle at 55℃. Expression levels were estimated using an iCycler iQ™ multi-color real-time PCR detection system (Bio-Rad Laboratories). The relative expression of the gene was then calculated as the ratio to a housekeeping gene using the Gene-x program (Bio-Rad Laboratories).
All experiments were conducted 3 times. The means±SDs were calculated from the data collected from individual mice. Significant differences were determined using the Student's t-test. Statistical analysis was conducted using the GraphPad Prism 4.0 software.
RESULTS
T. spiralis infection could not fully elicit a Th2 response in PAR2 KO mice
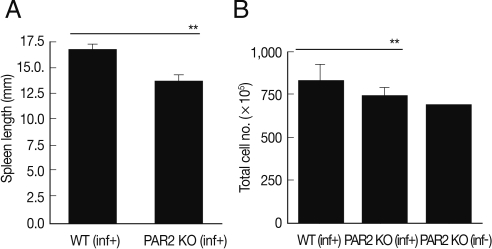
After sacrificing mice, we found that the length and size of the spleens of WT mice were increased significantly by
T. spiralis infection (
Fig. 1A). Although the numbers of splenocytes of PAR2 KO mice were increased slightly by
T. spiralis infection, the numbers of splenocytes of PAR2 KO mice were significantly smaller than those of the
T. spiralis-infected WT mice (
Fig. 1B). In order to evaluate the role of PAR2 in Th2 responses by parasite infections, we isolated lymphocytes from the spleen and MLNs obtained from the WT and PAR2 KO mice infected with
T. spiralis and analyzed the Th2 cytokine levels in these cells after stimulation with anti-CD3 antibodies and parasite total extracts.
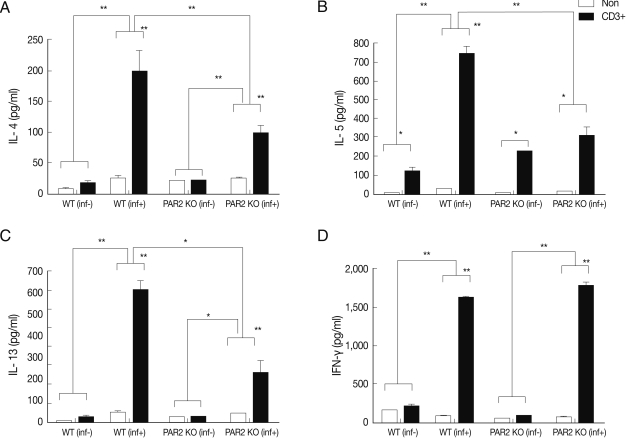
IL-4, IL-5, and IL-13 secretion levels in the spleen lymphocytes of WT mice were significantly increased by treatment with anti-CD3 antibodies, but these cytokines were not quite increased in the PAR2 KO mice (
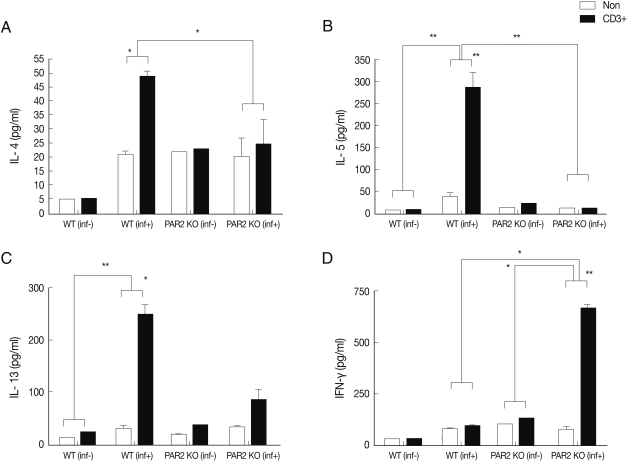
Fig. 2). The IL-4 secreting lymphocyte level increased significantly in the MLNs of WT mice after
T. spiralis infection, but these cells in the PAR2 KO mice were not increased (
Fig. 3A). Also, the IL-5 secreting lymphocyte level increased significantly only in the MLNs of WT mice but not in the PAR2 KO mice (
Fig. 3B). Slightly elevated levels of IL-13 secretion were also observed in the MLNs of both mice (
Fig. 3C). In addition, the IFN-γ secretion level of lymphocytes increased significantly in MLNs of PAR2KO mice compared with WT mice; however, there was no significant increase in the spleens of both mice (
Figs. 2D,
3D).
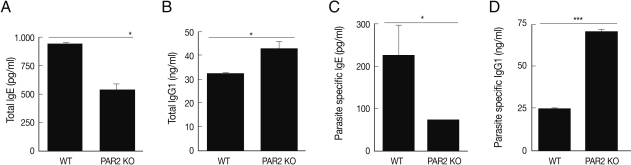
In an effort to gain insight into the role of PAR2 in IgE synthesis, we analyzed total and parasite-specific IgE (pg/ml) in the serum of WT and PAR2 KO mice infected with
T. spiralis. The total serum IgE of WT increased significantly by
T. spiralis infection, but those of PAR2 KO mice were significantly lower (
Fig. 4A). The parasite-specific serums IgE level was significantly increased as the consequence of
T. spiralis infection (
Fig. 4C). The total serum IgG1 level (ng/ml) of PAR2 KO mice were significantly higher than those noted in the WT mice (
Fig. 4B). In addition, parasite-specific IgG1 level in the PAR2 KO mice was significantly higher than those observed in WT mice (
Fig. 4D). There were no differences in the levels of total IgG2a levels (ng/ml) between WT and PAR2 KO mice (data not shown).
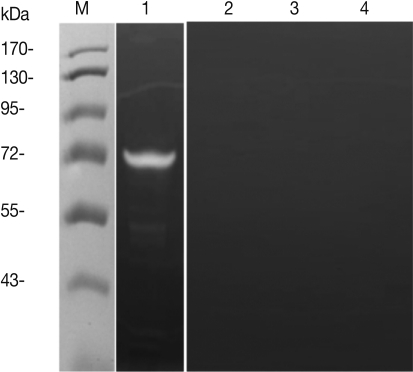
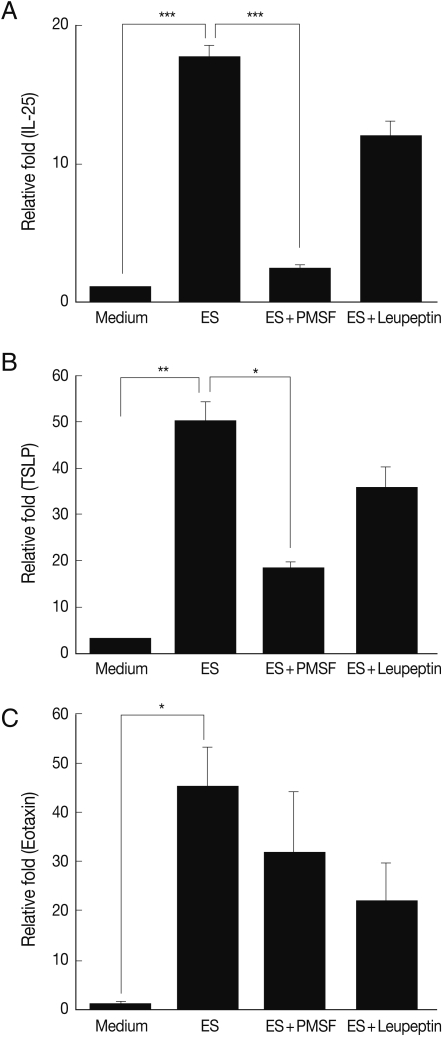
To investigate the protease activity of
T. spiralis ES proteins, we conducted a zymogram analysis. The results showed that the ES protein had many proteases, and their protease activity was inhibited by PMSF (serine protease inhibitor) and leupeptin (cystein protease inhibitor) pre-treatment, or by boiling (
Fig. 5). In order to determine whether or not
T. spiralis ES proteins could increase Th2 chemokine gene expression, ES proteins were administered to CT-26 mouse intestinal epithelial cells. After ES protein treatments, thymic stromal lymphopoietin (TSLP) and IL-25 gene expression of CT-26 cells increased significantly above the level of the control group (
Fig. 6A, 6B). However, the expressions of these Th2 chemokine genes were significantly inhibited by PMSF, but not by leupeptin. Also, we found that elevated eotaxin gene expression by ES protein treatment was slightly decreased after PMSF treatment, but this was not significant (
Fig. 6C).
DISCUSSION
In our study, we demonstrated that PAR2 was an initiator of the Th2 immune response against
T. spiralis larval infection. When PAR2 was deficient, the Th2 response did not fully develop. In general, the infection of mammals by helminthic parasites typically induces a type 2 immune response, which is broadly characterized by activation of eosinophils, basophils, and mast cells, as well as IgE production, and proliferation of T cells that secrete IL-4, IL-5, IL-9, IL-10, and IL-13 [
15]. Additionally, these immune responses could be readily observed in cases of
T. spiralis infection. However, until now, the mechanisms about Th2 response elevation against
T. spiralis infection remained unclear. It has been well established that viruses, bacteria, and protozoa could trigger a variety of pathogen-associated molecular patterns, such as the TLRs, present on antigen presenting cells. However, these receptors have proven to be difficult to fully explain in the context of innate immune responses against helminthic infections.
Some parasite-derived materials, particularly those from
Schistosoma, and certain phosphorylcholine-decorated glycans from filarial nematodes, could induce Th2 responses via TLR3 or TLR4 [
16-
18]. However, these factors are not sufficient to explain the initiation of the Th2 response against helminth infections. It has been well established that PAR, and particularly PAR2, was closely related with Th2 responses as the result of allergic inflammation (eosinophil recruitment, Th2 chemokine and cytokine increase) [
19,
20]. In addition, the Th2 response has been readily noted in the cases of helminthic infections. As previously mentioned, helminth proteases are crucial for maintenance of the helminth life cycle, particularly larval infection, digestion of host material, and molting. This may be closely related between helminthic infection and PAR2.
In our study, we observed that Th2 cytokine (IL-4, IL-5, and IL-13) production of splenocytes and lymphocytes in MLNs of PAR2 KO mice was significantly reduced as compared to WT mice (
Figs. 2,
3). In addition, we have demonstrated that the total IgE levels and parasite-specific IgE levels in the serum of PAR2 KO mice were slightly increased but the level was quite lower than those of WT mice (
Fig. 4). These results might be attributable to low IL-4 levels, which are crucial for activation of B cells and production of IgE. Interestingly, the levels of total and parasite-specific IgG1 in the serum of PAR2 KO mice were significantly higher than those of WT mice (
Fig. 4). We surmise that IgE could not be fully switched from IgG1 in PAR2 KO mice. All things considered, the Th2 responses in the infected PAR2 KO mice significantly lower than those seen in the infected WT mice.
What is the connecting factor in the correlation between PAR2 and Th2 responses? IL-25 (also known as IL-17E), a member of the IL-17 family, has been previously implicated in Th2 cell-mediated immunity [
21,
22]. Recently, IL-25 has also been implicated as one of the initiators of the Th2 responses [
23] and demonstrated to be expressed by mast cells [
24]. The transgenic overexpression of IL-25 by lung epithelial cells results in mucus production and airway infiltration of macrophages and eosinophils; conversely, the blockage of IL-25 reduces airway inflammation and Th2 cytokine production in an allergen-induced asthma model [
23,
25]. Additionally, the expression of TSLP, an IL-7-like cytokine, is associated with skin or bronchial epithelial cells, although the physiological inducers of TSLP expression have yet to be understood. TSLP has been demonstrated to activate DCs, which then subsequently prime naive T cells to express Th2 cytokines, result in precipitating allergic responses [
26,
27].
In addition, TSLP can function directly on T cells to promote Th2 differentiation [
26,
28]. Therefore, IL-25 and TSLP appear to play a pivotal role in provoking allergic inflammation, and particularly in Th2 allergic responses. In our study, IL-25 and TSLP gene expressions of intestinal epithelial cells increased significantly as the result of stimulation with
T. spiralis ES protein, but these gene expressions were significantly reduced by serine protease inhibitors, not cystein protease inhibitors (
Fig. 6). Therefore,
T. spiralis ES protein could activate PAR2 by serine protease activity. Recently Kouzaki et al. [
29] reported that proteases induce production of TSLP through PAR2, and TSLP triggers dendritic cell-mediated Th2 type inflammation.
Trichinella also excreted and/or secreted various kinds of proteases for invasion and digestion of host tissues [
11-
13], and these proteases might elicit TSLP via PAR2.
In conclusion, PAR2 may play a critical role in initiation of Th2 responses against T. spiralis infection, particularly with Th2 cytokines and parasite-specific IgE production via IL-25 and TSLP activation.
ACKNOWLEDGEMENTS
This work was supported by the Bio-Scientific Research Grant funded by the Pusan National University (PNU, Bio-Scientific Research Grant) (PNU-2008-101-207).
References
- 1. Devlin MG, Gasser RB, Cocks TM. Initial support for the hypothesis that PAR2 is involved in the immune response to Nippostrongylus brasiliensis in mice. Parasitol Res 2007;101:105-109.
- 2. Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol 2003;3:984-993.
- 3. McKerrow JH, Caffrey C, Kelly B, Loke P, Sajid M. Proteases in parasitic diseases. Annu Rev Pathol 2006;1:497-536.
- 4. Ghaemmaghami AM, Gough L, Sewell HF, Shakib F. The proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: allergen-induced Th2 bias determined at the dendritic cell level. Clin Exp Allergy 2002;32:1468-1475.
- 5. Tovey ER, Chapman MD, Platts-Mills TA. Mite faeces are a major source of house dust allergens. Nature 1981;289:592-593.
- 6. Rosario NA. Total IgE in respiratory allergies and infections by intestinal parasites. J Pediatr (Rio J) 2007;83:92-93. author reply 93-94.
- 7. Traynelis SF, Trejo J. Protease-activated receptor signaling: New roles and regulatory mechanisms. Curr Opin Hematol 2007;14:230-235.
- 8. Ossovskaya VS, Bunnett NW. Protease-activated receptors: Contribution to physiology and disease. Physiol Rev 2004;84:579-621.
- 9. Holzhausen M, Spolidorio LC, Vergnolle N. Role of protease-activated receptor-2 in inflammation, and its possible implications as a putative mediator of periodontitis. Mem Inst Oswaldo Cruz 2005;100(suppl 1):177-180.
- 10. Fabre MV, Beiting DP, Bliss SK, Appleton JA. Immunity to Trichinella spiralis muscle infection. Vet Parasitol 2009;159:245-248.
- 11. Cwiklinski K, Meskill D, Robinson MW, Pozio E, Appleton JA, Connolly B. Cloning and analysis of a Trichinella pseudospiralis muscle larva secreted serine protease gene. Vet Parasitol 2009;159:268-271.
- 12. Liu MY, Wang XL, Fu BQ, Li CY, Wu XP, Le Rhun D, Chen QJ, Boireau P. Identification of stage-specifically expressed genes of Trichinella spiralis by suppression subtractive hybridization. Parasitology 2007;134:1443-1455.
- 13. Park HK, Chang SW, Kang SW, Cho MK, Choi SH, Hong YC, Lee YS, Jeong HJ, Yu HS. Expressed sequence tags of Trichinella spiralis muscle stage larvae. Korean J Parasitol 2008;46:59-63.
- 14. Dixon H, Blanchard C, Deschoolmeester ML, Yuill NC, Christie JW, Rothenberg ME, Else KJ. The role of Th2 cytokines, chemokines and parasite products in eosinophil recruitment to the gas-trointestinal mucosa during helminth infection. Eur J Immunol 2006;36:1753-1763.
- 15. Diaz A, Allen JE. Mapping immune response profiles: The emerging scenario from helminth immunology. Eur J Immunol 2007;37:3319-3326.
- 16. Goodridge HS, Marshall FA, Else KJ, Houston KM, Egan C, Al-Riyami L, Liew FY, Harnett W, Harnett MM. Immunomodulation via novel use of TLR4 by the filarial nematode phosphorylcholine-containing secreted product, ES-62. J Immunol 2005;174:284-293.
- 17. Thomas PG, Carter MR, Atochina O, Da'Dara AA, Piskorska D, McGuire E, Harn DA. Maturation of dendritic cell 2 phenotype by a helminth glycan uses a Toll-like receptor 4-dependent mechanism. J Immunol 2003;171:5837-5841.
- 18. Aksoy E, Zouain CS, Vanhoutte F, Fontaine J, Pavelka N, Thieblemont N, Willems F, Ricciardi-Castagnoli P, Goldman M, Capron M, Ryffel B, Trottein F. Double-stranded RNAs from the helminth parasite Schistosoma activate TLR3 in dendritic cells. J Biol Chem 2005;280:277-283.
- 19. Lee KE, Kim JW, Jeong KY, Kim KE, Yong TS, Sohn MH. Regulation of German cockroach extract-induced IL-8 expression in human airway epithelial cells. Clin Exp Allergy 2007;37:1364-1373.
- 20. Buddenkotte J, Stroh C, Engels IH, Moormann C, Shpacovitch VM, Seeliger S, Vergnolle N, Vestweber D, Luger TA, Schulze-Osthoff K, Steinhoff M. Agonists of proteinase-activated receptor-2 stimulate upregulation of intercellular cell adhesion molecule-1 in primary human keratinocytes via activation of NF-kappa B. J Invest Dermatol 2005;124:38-45.
- 21. Hurst SD, Muchamuel T, Gorman DM, Gilbert JM, Clifford T, Kwan S, Menon S, Seymour B, Jackson C, Kung TT, Brieland JK, Zurawski SM, Chapman RW, Zurawski G, Coffman RL. New IL-17 family members promote Th1 or Th2 responses in the lung: In vivo function of the novel cytokine IL-25. J Immunol 2002;169:443-453.
- 22. Fort MM, Cheung J, Yen D, Li J, Zurawski SM, Lo S, Menon S, Clifford T, Hunte B, Lesley R, Muchamuel T, Hurst SD, Zurawski G, Leach MW, Gorman DM, Rennick DM. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity 2001;15:985-995.
- 23. Angkasekwinai P, Park H, Wang YH, Wang YH, Chang SH, Corry DB, Liu YJ, Zhu Z, Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med 2007;204:1509-1517.
- 24. Ikeda K, Nakajima H, Suzuki K, Kagami S, Hirose K, Suto A, Saito Y, Iwamoto I. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood 2003;101:3594-3596.
- 25. Ballantyne SJ, Barlow JL, Jolin HE, Nath P, Williams AS, Chung KF, Sturton G, Wong SH, McKenzie AN. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J Allergy Clin Immunol 2007;120:1324-1331.
- 26. Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med 2005;202:829-839.
- 27. Miyata M, Hatsushika K, Ando T, Shimokawa N, Ohnuma Y, Katoh R, Suto H, Ogawa H, Masuyama K, Nakao A. Mast cell regulation of epithelial TSLP expression plays an important role in the development of allergic rhinitis. Eur J Immunol 2008;38:1487-1492.
- 28. Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol 2007;178:1396-1404.
- 29. Kouzaki H, O'Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol 2009;183:1427-1434.
Fig. 1Comparison of spleen enlargement by T. spiralis infection between PAR2 KO mice and WT mice. The length of spleens was calculated by a ruler (A). The number of splenocytes was calculated using a hemocytometer after spleen cell isolation (B). inf(+), T. spiralis-infected; inf(-), T. spiralis-uninfected (**P<0.01).
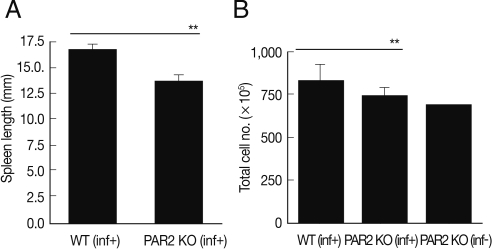
Fig. 2Cytokines production of splenocytes in PAR2 KO and WT mice. inf(+), T. spiralis-infected; inf(-), T. spiralis-uninfected; Non, non-stimulation; CD3+, anti-CD3 antibody stimulation (*P<0.05, **P<0.01).
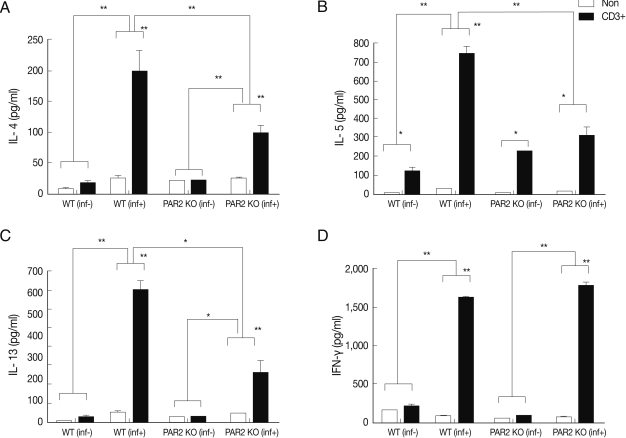
Fig. 3Cytokines production of MLN lymphocytes in PAR2 KO and WT mice. inf(+), T. spiralis-infected; inf(-), T. spiralis-uninfected; Non, non-stimulation; CD3+, anti-CD3 stimulation (*P<0.05, **P<0.01).
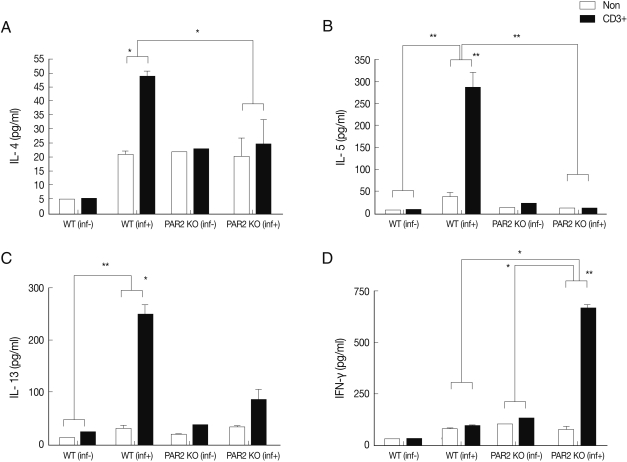
Fig. 4Comparison of total immunoglobulins and anti-T. spiralis-specific immunoglobulins level between PAR2 KO and WT mice (*P<0.05, ***P<0.001).
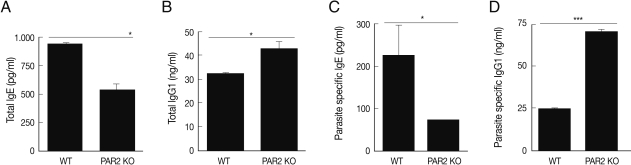
Fig. 5Evaluation of protease activity of T. spiralis excretory-secretory proteins. Protease activity of ES protein (A). M, molecular marker; Lane 1, ES protein of T. spiralis; Lane 2, boiled ES protein; Lane 3, PMSF pre-treated ES protein; Lane 4, leupeptin pre-treated ES protein.
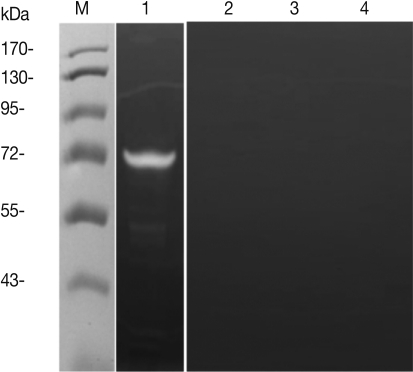
Fig. 6Th2 chemokine gene expression of CT-26 cells after T. spiralis excretory-secretory protein treatment. ES, T. spiralis ES protein; ES+PMSF, PMSF pre-treated ES protein; ES+Leupeptin, leupeptin pre-treated ES protein (*P<0.05, **P<0.01, ***P<0.001).
Table 1.Primers used for real-time PCR
Table 1.
|
Primer |
Sequence |
|
GAPDH-for*
|
5´ - TACCCCCAATGTGTCCGTC - 3´ |
|
GAPDH-rev†
|
5´ - AAGAGTGGGAGTTGCTGTTGAAG - 3´ |
|
Eotaxin-for |
5´ - GCGCTTCTATTCCTGCTGCTCACGG - 3´ |
|
Eotaxin-rev |
5´ - GTGGCATCCTGGACCCACTTCTTC - 3´ |
|
TSLP-for |
5´ - GGAGATTTGAAAGGGGCTAAG - 3´ |
|
TSLP-rev |
5´ - TGGGCAGTGGTCATTGAG - 3´ |
|
IL-25-for |
5´ - TGGCAATGATCGTGGGAACC - 3´ |
|
IL-25-rev |
5´ - GAGAGATGGCCCTGCTGTTGA - 3´ |