Abstract
Toxoplasma gondii penetrates all kinds of nucleated eukaryotic cells but modulates host cells differently for its intracellular survival. In a previous study, we found out that serine protease inhibitors B3 and B4 (SERPIN B3/B4 because of their very high homology) were significantly induced in THP-1-derived macrophages infected with T. gondii through activation of STAT6. In this study, to evaluate the effects of the induced SERPIN B3/B4 on the apoptosis of T. gondii-infected THP-1 cells, we designed and tested various small interfering (si-) RNAs of SERPIN B3 or B4 in staurosporine-induced apoptosis of THP-1 cells. Anti-apoptotic characteristics of THP-1 cells after infection with T. gondii disappeared when SERPIN B3/B4 were knock-downed with gene specific si-RNAs transfected into THP-1 cells as detected by the cleaved caspase 3, poly-ADP ribose polymerase and DNA fragmentation. This anti-apoptotic effect was confirmed in SERPIN B3/B4 overexpressed HeLa cells. We also investigated whether inhibition of STAT6 affects the function of SERPIN B3/B4, and vice versa. Inhibition of SERPIN B3/B4 did not influence STAT6 expression but SERPIN B3/B4 expression was inhibited by STAT6 si-RNA transfection, which confirmed that SERPIN B3/B4 was induced under the control of STAT6 activation. These results suggest that T. gondii induces SERPIN B3/B4 expression via STAT6 activation to inhibit the apoptosis of infected THP-1 cells for longer survival of the intracellular parasites themselves.
-
Key words: Toxoplasma gondii, macrophage, SERPIN B3, B4, STAT6, anti-apoptosis
INTRODUCTION
Toxoplasma gondii, an intracellular protozoan parasite, infects a wide range of warm-blooded animals world-widely, including up to 30-70% of the human population [
1,
2]. Normal human infection is most asymptomatic, but those of immunodeficient humans or fetuses from primarily infected mothers and newborns lead to a life-threatening serious disease or death [
3].
Macrophages are in the front line of host defense against various pathogenic infections. Although macrophages possess microbicidal and immunoactivating properties, several protozoan parasites, including
T. gondii, can evade, enter, survive, and grow within this potent host defense cell [
4-
8]. Intracellular parasite
T. gondii prolonged its parasitism by manipulation of host cellular defense or apoptosis of host cells.
T. gondii blocks host cell apoptosis by direct interference with those molecules related to the signaling pathway of apoptosis, such as caspase cascades, PARP [
9,
10], or mitochondria-dependent programmed cell death (PCD) [
11], but the corresponding molecules involved in the signaling pathway of anti-apoptotic mechanism have not been clarified. Even some inhibitors of protein synthesis potentiate staurosporine-induced PCD, it is clear that PCD does not require the synthesis of new proteins, whereas the activation of the death program by some other inducers of PCD clearly does so [
12]. Caspase cleavage of PARP blocks DNA repair and thereby facilitates apoptosis, so the PARP cleavage is a useful indicator of PCD and substrate of caspases in vitro and in vivo.
In our previous study, we found that serine protease inhibitors, such as SPINT2, SERPIN B3, B4, and B13 were significantly expressed in
T. gondii-infected macrophages [
13]. Among them, SERPIN B3 and B4 are highly homologous each other and also named as squamous cell carcinoma antigens (SCCA) 1 and 2, respectively. SERPIN has ability to protect from tumor necrosis factor-mediated apoptosis [
14] and calpain activation [
15]. In the present study, we present the evidences for those up-regulated SERPINs by the infection of
T. gondii to the protective roles in apoptotic signaling pathway with inhibition of casapase 3, PARP activation, and DNA fragmentation. This anti-apoptotic action of SERPIN B3/B4 may favor
T. gondii to prolong successfully its parasitism in host cells.
MATERIALS AND METHODS
Parasite and host cells
The RH strain of T. gondii was maintained by peritoneal passages in BALB/c mice. Prior to use, tachyzoites were purified by centrifugation over 40% Percoll (Amersham Pharmacia Biotech, Uppsala, Sweden) in PBS. Human acute monocytic leukemia cells (THP-1, ATCC TIB-202, American Type Culture Collection, Manassas, Virginia, USA) and HeLa (ATCC CCL2) cells were cultured in minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS). THP-1 cells were used as macrophages after induction to differentiate with 0.25 µM phorbol myristate acetate (PMA) for 48 hr. A549 cells and Jurkat T cells (E6-1) were grown in RPMI 1640 medium supplemented with 25 mM HEPES, 50 mg/L gentamicin sulphate, and 10% (v/v) heat-inactivated FBS at 37℃ in a humidified 5% CO2 atmosphere.
RT-PCR of SERPINs in various types of cells
Various types of cells, i.e., HeLa, A549, Jurkat T, U937, and THP-1 (monocytes and differentiated macrophages), were tested for expression of SERPIN B3 and B4 24 hr after T. gondii infection. First-strand cDNA was synthesized from 2 µg of total RNA and reacted with 10 µM primer sets for SERPIN B3, 5'-GAT GAG GCA ATA CAC ATC TTT TCA-3' and 5'-CAG CAG TGA GTT TCT CTT CAA GC-3'; SERPIN B4, 5'-CAA AGG CAA AGA TCT AAG CAT GA-3' and 5'-CAA TTT CTC AGC AGT GAG TTT CTC-3'; and control β-actin (X00351), 5'-GCA CCC AGC ACA ATG AAG A-3' and 5'-CGA TCC ACA CGG AGT ACT TG-3'.
Effects of si-SERPIN B3 and B4 RNA or si-STAT6 RNA
SERPIN B3 or B4 was knock-downed by direct transfection with various site specific si-RNAs (
Table 1) and interference with transcribed STAT6 mRNA with 5'-AAG CAG GAA GAA CUC AAG UUU TT-3' and 5'-AAA CUU GAG UUC UUC CUG CUU TT-3' [
13]. Cells cultured on round coverslips in a 24-well plate were transfected with a premix of 6
pmole siRNA and Lipofectamin RNAi MAX (Invitrogen, Carlsbad, California, USA) in Opti-MEM for 6 hr. After washing with MEM, cells were further incubated for 24 hr.
THP-1 cells and HeLa cells were infected with T. gondii at parasite-to-host cell ratio of 10:1 and incubated for 16 hr and differentiate with 0.25 µM PMA for 24 hr. Before induction of apoptosis, THP-1 cells and HeLa cells were washed 3 times in RPMI 1640 or EMEM to remove extracellular T. gondii. To induce apoptosis, staurosporine of 500 nM was treated for 8 hr to host cells.
Western blot
Proteins were resolved in 12% SDS-PAGE and transferred onto nitrocellulose (NC) sheets. The NC sheets blocked with 5% skim milk in PBS containing 0.05% Tween-20 were incubated with 1:1,000 diluted rabbit anti-human caspase 3, PARP, β-actin antibodies (Cell Signaling Technology, Danvers, Massachusetts, USA) and SCCA1/2 antibody (Santa Cruz Biotechnology, Santa Cruz, California, USA), and then with 1:5,000 diluted HRP-conjugated goat anti-rabbit IgG antibody (Santa Cruz Biotechnology). They were soaked in enhanced chemiluminescence solution (Amersham Phamarcia Biotech) for 1 min and then the chemiluminescent images were obtained in Bio-Rad Gel Doc XR+ (Bio-Rad Lab., Hercules, California, USA).
DNA fragmentation
To induce apoptosis, macrophages were treated with staurosporine (500 nM; Sigma Chemical Co., Rockville, Maryland, USA) for 8 hr after T. gondii infection 24 hr earlier. The cells were harvested by centrifugation and washed with cold PBS. DNA was extracted using TakaRa kit (MK600, Otsu, Shiga, Japan). The DNA samples were separated by electrophoresis on a 2% agarose gel and visualized under UV by ethidium bromide.
Cloning and co-transfection of SERPIN B3 and B4 into HeLa cells
The first strand cDNA was synthesized using the Powerscript reverse transcriptase (BD Biosciences Clontech, Mountain View, California, USA) from 1 µg of total RNA which was isolated from A549 cells using TRIzol reagent (Invitrogen). For the SERPIN B3, PCR was performed with a forward primer of 5'-atg aat tca c tc agt gaa gcc aac acc aag ttc atg ttc gac-3' and reverse primer of 5'-cta cgg gga tga gaa tct gaa ata gaa gag gat-3' to amplify 1,173 bp of Homo sapiens A53A squamous cell carcinoma antigen (SCCA) 1 gene (AY245781) and for SERPIN B4 (AY245782), a forward primer 5'-atg aat tca ctc agt gaa gcc aac acc aag ttc atg ttc ggt-3' and reverse primer 5'-cta cgg ggg tga gaa tct gaa ata gaa gag gat-3' were used to amplify 1,173 bp of Homo sapiens 1BP1 SCCA 2 (AY245782). They were inserted into red fluorescent plasmid pDsRed2-N1 expression vector (BD Biosciences, Clontech). Transient co-transfection of pDsRed2-N1-SERPIN B3 and pDsRed2-N1-SERPIN B4 into HeLa cells were achieved using calcium phosphate co-precipitation method. The day before transfection, 5×104 cells were seeded into 24-well culture plates in fresh medium. Plasmid DNA (1-2 µg) was diluted in 42 µl of H2O, mixed with 7 µl of 2.0 M CaCl2 and added by drops to 50 µl of 2×HeBS (280 mM NaCl, 1.5 mM Na2HPO4, and 50 mM HEPES, pH 7.05). After 20-min incubation at room temperature, the mixture was added to the cells. After 24 hr, cells were washed and infected with RH tachyzoites of 1×108 per ml.
RESULTS
SERPIN B3 and B4 were significantly induced in T. gondii-infected macrophages
The intracellular
T. gondii manipulated its host cell genome such that the expression of SPINT2, SERPIN B3, B4, and B13 were consistently increased over 3-folds among 17 SERPINs filtered in the microarray analysis of macrophages following infection with
T. gondii [
13]. In this study, we observed preliminarily the expression of SERPIN B3 and B4 in several cell types, which showed differences in the induction between normal and
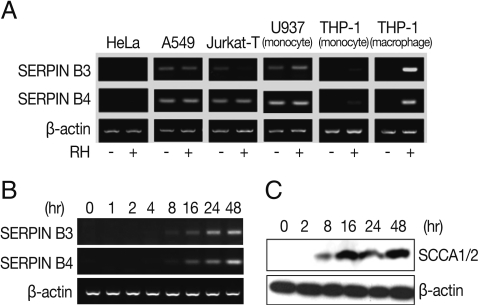
T. gondii infected cells (
Fig. 1A). In HeLa cells, both SERPIN B3 and B4 were not expressed regardless of
T. gondii infection and, in A549, Jurkat-T, and U937 cells, SERPIN B3 was expressed as smudges and SERPIN B4 expressed fully but the expressions were still both in normal and infected cells also. In case of THP-1 cells, both SERPIN B3 and B4 were not expressed in normal cells but those were significantly increased in PMA-induced THP-1 cells, i.e., macrophages after
T. gondii infection.
The induction of SERPIN B3 and B4 started as early as 8 hr post-infection with
T. gondii, increased gradually up to 24 hr and then maintained thereafter in transcriptional level (
Fig. 1B) and translational level as blotted by anti-SCCA 1/2 antibody (
Fig. 1C).
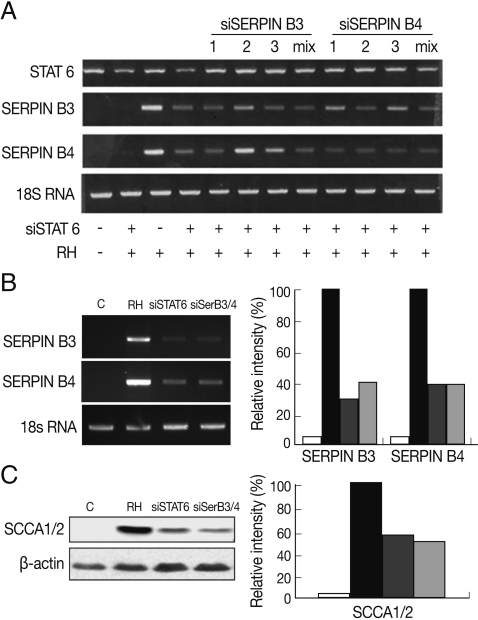
To correlate the interaction between SERPIN B3 and B4 and STAT6 in macrophages infected with
T. gondii, we first designed and tested various small interfering (si-) RNA of SERPIN B3 or B4 (
Table 1). Among the SERPIN B3-1~3 and SERPIN B4-1~3 si-RNA, si-SERPIN B3-1 and si-SERPIN B4-2 RNA could effectively interfere with the transcriptional level of SERPIN B3 and B4, respectively (
Fig. 2A), without affecting the STAT6 transcription level. The si-SERPIN B3/B4 RNA mix (mixture of si-SERPIN B3-1 and si-SERPIN B4-2 RNA) was selected and applied, which resulted in the significant knock-down of the mRNA transcription of SERPIN B3 or B4 with si-SERPIN B3/B4 RNA mix by 62% of SERPIN B3 and 67% of SERPIN B4. Treatment of si-STAT6 RNA also influenced the transcription of SERPIN B3 by 71% and SERPIN B4 by 67%, respectively (
Fig. 2B). The protein translation of SERPIN B3 or B4 was accordingly reduced with si-SERPIN B3/B4 RNA mix concordance (51%) or si-STAT6 RNA transfection (46%) (
Fig. 2C).
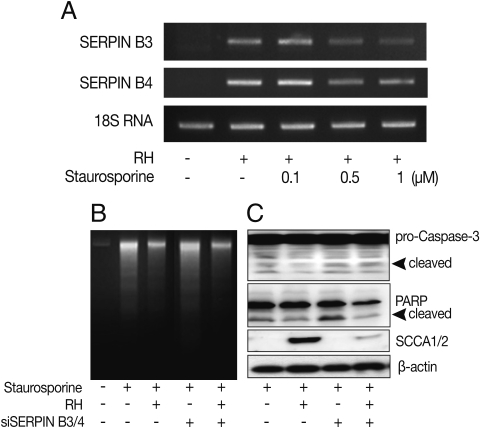
To clarify the function of the induced SERPIN B3 and B4 in the apoptosis of host cells, we promoted the apoptosis of macrophages with staurosporine which is a well known inducer of apoptosis in mammalian cells [
16]. The expression of SERPIN B3 or B4 was maintained under 1 µM of staurosporine treatment (
Fig. 3A).
T. gondii-infected macrophages showed significantly decreased apoptotic cell death detected by DNA fragmentation (
Fig. 3B), activation of caspase 3 and PARP cleavage (
Fig. 3C) upon treatment of staurosporine (0.5 µM for 8 hr) compared with uninfected cells. Inhibited apoptotic characteristics of macrophages infected with
T. gondii were significantly reduced when SERPIN B3 and B4 were knock-downed with gene specific si-RNA transfection to macrophages.
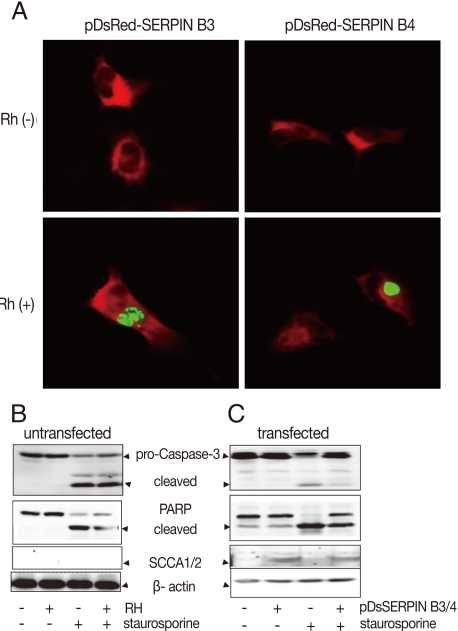
To confirm the function of SERPIN B3/B4 (SCCA1/2) in apoptosis, the carcinoma cell line HeLa, which showed no background expression of SERPIN B3 and B4 in normal and in
T. gondii-infected cells (
Fig. 1A), was co-transfected with the mammalian expression vectors pDsRed-N1 containing cDNA for SERPINB3 or B4 coding regions. The expression of SERPIN B3/B4 in transfected cells was confirmed using fluorescence microscope and western blot with SCCA1/2 antibody which reacts with SERPIN B3 and B4 proteins. The expression of SERPIN B3/B4 was detected in infected or uninfected HeLa cells with
T. gondii (RH,
Fig. 4A). HeLa cells devoid of SERPIN B3/B4 expression could not protect staurosporine-induced apoptosis although
T. gondii was infected or not (
Fig. 4B). But SERPIN B3/B4 co-transfected HeLa cells showed strongly reduced apoptotic characteristics by caspase3 activation and PARP cleavage (
Fig. 4C).
DISCUSSION
The cross talk of
T. gondii with various macrophage populations has been reported, wherein
T. gondii prolonged their survival in macrophages by preventing nuclear translocation of transcription factor NF-kB [
7], inhibiting the production of proinflammatory cytokines [
17] and reducing the expression of inducible nitric oxide synthase [
18]. Genome and proteome analysis [
19,
20] of
T. gondii-infected host cells have enabled to investigate how intracellular parasite
T. gondii manipulates its host cells at DNA or post-transcriptional levels. Furthermore, modulation of host cell apoptosis is an important feature of
T. gondii infections.
In our previous study using microarray of host cells infected with
T. gondii, SERPINs were significantly expressed in
T. gondii-infected macrophages [
13]. Especially, SCCA1 (SERPIN B3) and SCCA2 (SERPIN B4) were highly induced in macrophages after
T. gondii infection. With these results, we speculate that intracellular
T. gondii may exert to manipulate the expression of SERPIN B3 or B4 by macrophages for its intracellular survival through controlling the fate of host cells. There are some evidences that SERPINs protect from tumor necrosis factor-mediated apoptosis by reducing the DNA fragmentation [
21], inactivating caspase and/or upstream proteases [
22], and overexpression [
14].
Although inhibition of SERPIN B3 or B4 did not affect the expression of transcription factor STAT6, STAT6 inhibition significantly affects SERPIN B3 and B4 expressions, especially SERPIN B3. These results confirmed our previous findings that STAT6 activation by
T. gondii infection induces the expression of Th2 C-C chemokine ligands and B clade serine protease inhibitors in macrophages [
13]. The SERPIN B3 and B4 may participate in the downstream of the signaling molecules from autonomous STAT6 activation by
T. gondii infection independent of IL-4 in macrophages. The up-regulation of SCCA1/2 genes via host transcription factor STAT6 in
T. gondii-infected macrophages is possibly an important mechanism in
T. gondii-mediated blockage of apoptosis of infected host cells.
In our study, the inhibition of SCCA1/2 expression resulted in host cell's failure of the protection against apoptosis signal pathway, such as caspase 3 activation, PARP cleavage, and DNA fragmentation. Furthermore, the overexpression of SCCA1/2 in HeLa cells significantly reduced the activation of caspase-3 induced by sataurosporine treatment, which suggested that SCCA1/2 may inhibit a protease upstream from these caspases. Our present results demonstrated that a method to suppress the apoptosis of macrophages by
T. gondii via induction of SERPIN B3 and B4 so that various pathways of apoptosis maybe differentially regulated by various protease inhibitors, such as plasminogen activator inhibitor type 2 [
23] and poxvirus-encoded serpin [
24] which target different caspases [
25].
In summary, our results are the first reports that T. gondii modulates the expression of SERPIN B3 and B4 as products of gamma-associated sequence (GAS) of activated STAT6 in infected macrophages, which may function as cellular factors involved in the regulation of apoptosis pathway of the professional phagocytic macrophages as a survival mechanism for its parasitism.
ACKNOWLEDGMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0073383).
References
- 1. Dubey JP. Toxoplasmosis-a waterborne zoonosis. Vet Parasitol 2004;126:57-72.
- 2. Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animal to humans. Int J Parasitol 2000;30:1217-1258.
- 3. Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet 2004;363:1965-1976.
- 4. Reiner NE. Altered cell signaling and mononuclear phagocyte deactivation during intracellular infection. Immunol Today 1994;15:374-381.
- 5. Hall BF, Webster P, Ma AK, Joiner KA, Andrew NW. Desialylation of lysosomal membrane glycoproteins by Trypanosoma cruzi: a role for the surface neuraminidase in facilitating parasite entry into the host cell cytoplasm. J Exp Med 1992;176:313-325.
- 6. Rittig MG, Bogdan C. Leishmania-host cell interaction: complexities and alteration views. Parasitol Today 2000;16:292-297.
- 7. Butcher BA, Kim L, Johnson PF, Denker EY. Toxoplasma gondii tachyzoites inhibit proinflammatory cytokine induction in infected macrophages by preventing nuclear translocation of the transcription factor NF-kB. J Immunol 2001;167:2193-2201.
- 8. Bogdan C, Rollinghoff M. How do protozoan parasites survive inside macrophages? Parasitol Today 1999;15:22-28.
- 9. Lüder CG, Gross U, Lopes MF. Intracellular protozoan parasites and apoptosis: diverse strategies to modulate parasite-host interactions. Trends Parasitol 2001;17:480-486.
- 10. Goebel S, Gross U, Lüder C. Inhibition of host cell apoptosis by Toxoplasma gondii is accompanied by reduced activation of the caspase cascade and alterations of poly(ADP-ribose) polymerase expression. J Cell Sci 2001;114:3495-3505.
- 11. Carmen JC, Hardi L, Sinai AP. Toxoplasma gondii inhibits ultraviolet light-induced apoptosis through multiple interactions with the mitochondrion-dependent programmed cell death pathway. Cell Microbiol 2006;8:301-315.
- 12. Duke RC, Cohen JJ. IL-2 addiction: Withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res 1986;5:289-299.
- 13. Ahn HJ, Kim JY, Ryu KJ, Nam HW. STAT6 activation by Toxoplasma gondii infection induces the expression of Th2 C-C chemokine ligands and B clade serine protease inhibitors in macrophage. Parasitol Res 2009;105:1445-1453.
- 14. Takeda A, Kajiya A, Iwasawa A, Nakamura Y, Hibino T. Aberrant expression of serpin squamous cell carcinoma antigen 2 in human tumor tissues and cell lines: evidence of protection from tumor necrosis factor mediated apoptosis. Biol Chem 2002;383:1231-1236.
- 15. Tonnetti L, Netzel-Arnett S, Darnell GA, Hayes T, Buzza MS, Anglin IE, Suhrbier A, Antalis TM. SerpinB2 protection of retinoblastoma protein from calpain enhances tumor cell survival. Cancer Res 2008;68:5648-5657.
- 16. Jacobsen MD, Weil M, Raff MC. Role of Ced-3/ICE-family proteases in staurosporine-induced programmed cell death. J Cell Biol 1996;133:1041-1051.
- 17. Butcher BA, Denkers EY. Mechanism of entry determines the ability of Toxoplasma gondii to inhibit macrophage proinflammatory cytokine production. Infect Immun 2002;70:5216-5224.
- 18. Lüder CG, Algner M, Lang C, Bleicher N, Gross U. Reduced expression of the inducible nitric oxide synthethase after infection with Toxoplasma gondii facilitates parasite replication in activated murine macrophages. Int J parasitol 2003;33:833-844.
- 19. Blader IJ, Manger ID, Boothroyd JC. Microarray analysis reveals previously unknown changes in Toxoplasma gondii-infected human cells. J Biol Chem 2001;276(26):24223-24231.
- 20. Saeij JP, Coller S, Boyle JP, Jerome ME, White MW, Boothroyd JC. Toxoplasma co-opts host gene expression by injection of a polymorphic kinase homologue. Nature 2007;445(7125):324-327.
- 21. McGettrick AF, Barnes RC, Worrall DM. SCCA2 inhibits TNF-mediated apoptosis in transfected HeLa cells. Eur J Biochem 2001;268:5868-5875.
- 22. Suminami Y, Nagashima S, Vujanovic NL, Hirabayashi K, Kato H, Whiteside TL. Inhibition of apoptosis in human tumour cells by the tumour associated serpin, SCC antigen-1. Br J Cancer 2000;82:981-989.
- 23. Gan H, Newman GW, Remold HG. Plasminogen activator inhibitor type2 prevents programmed cell death of human macrophages infected with Mycobacterium avium, serovar 4. J Immunol 1995;155:1304-1315.
- 24. Tewari M, Telford WG, Miller RA, Dixit VM. CrmA, a poxvirus-encoded serpin, inhibits cytotoxic T-lymphocyte mediated apoptosis. J Biol Chem 1995;270:22705-22708.
- 25. Enari M, Hug H, Nagata S. Involvement of an ICE-like protease in Fas-mediated apoptosis. Nature 1995;375:78-81.
Fig. 1Expression of SERPIN B3 and B4 in various host cells. Expression of mRNA of SERPIN B4 and B4 in T. gondii infected or uninfected host cells (A). HeLa (cervix adenocarcinoma, epithelial), A549 (lung carcinoma, epithelial), Jurkat T (acute T cell lymphoma, T lymphocyte, lymphoblast), U937 (histiocytic lymphoma, histocyte, monocyte), THP-1 (peripheral blood, acute monocytic leukemia or macrophage) were tested. RT-PCR (B) and western blot (C) of induced SERPIN B3 and B4 in macrophage-differentiated THP-1 cells by time course. SCCA1/2 indicates SERIPN B3/B4 detectable antibody.
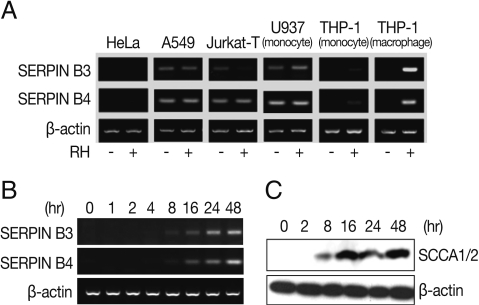
Fig. 2Inhibitory relationship between SERPIN B3 or B4 and STAT6. Inhibitory effects of various si-SERPIN B3 or B4 RNAs or si-STAT6 in transcriptional level of SERPIN B 3 and B4 genes (A). Inhibitory effects of si-STAT6 and si-SERPIN B3/B4 mix in m-RNA level (B) and protein level of SERPIN B3 and B4 (C). Blank bar indicates control host cells, black bar by T. gondii infection only, dark grey bar with siSTAT6 under T. gondii infection, and bright grey bar with siSERPIN B3/B4 under T. gondii infection, respectively.
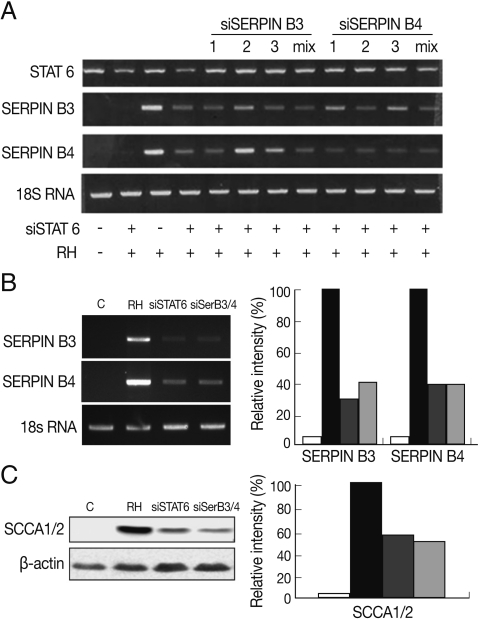
Fig. 3Anti-apoptotic effects of SERPIN B3/B4 and T. gondii infection in macrophages after induction of apoptosis by staurosporine. The transcriptional level of SERPIN B3 and B4 was affected by the concentration of staurosporine (A). DNA fragmentation (B), activation of caspase-3 and cleavage of PARP (B) were measured in macrophages. SCCA1/2 indicates SERIPN B3/B4 detectable antibody and β-actin was used as control.
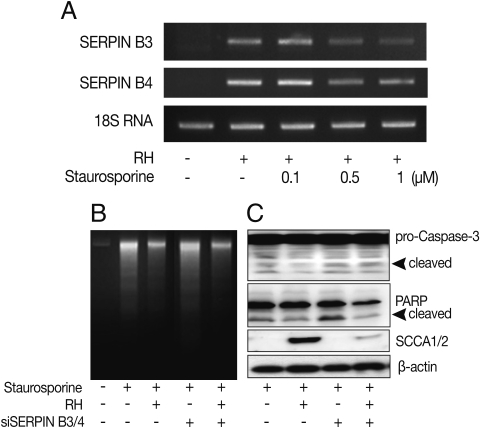
Fig. 4Effects of anti-apoptosis in HeLa cells by expression of SERPIN B3 and B4. Over-expression of SERPIN B3 or B4 in macrophages was confirmed in fluorescent photographs (A) of which RH(+) indicates T. gondii infection. Activation of caspase-3, cleavage of PARP, and SCCA1/2 expression in untransfected HeLa cells (B) and in transfected HeLa cells (C). pDsSERPIN B3/4 indicates pDsRed-SERPIN B3 and pDsRed-SERPIN B4 co-transfected HeLa cells.
Table 1.The si-RNAs designed and tested for inhibition of SERPIN B3 and B4
Table 1.
|
SERPINB3-1 |
(RNA) |
-AUA |
UCC |
ACC |
AUU |
CCC |
AUG |
GUU |
CUC |
A |
|
(RNA) |
-UGA |
GAA |
CCA |
UGG |
GAA |
UGG |
UGG |
AUA |
A |
|
SERPINB3-2 |
(RNA) |
-UUA |
UUA |
AAU |
UUC |
UUC |
UCC |
CAC |
UGC |
C |
|
(RNA) |
-GGC |
AGU |
GGG |
AGA |
AGA |
AAU |
UUA |
AUA |
A |
|
SERPINB3-3 |
(RNA) |
-UGG |
ACU |
UGU |
AUG |
UAU |
UCU |
UGU |
UUG |
G |
|
(RNA) |
-CCA |
AAC |
AAG |
AAU |
ACA |
UAC |
AAG |
UCC |
A |
|
SERPINB4-1 |
(RNA) |
-AAA |
UUU |
AUU |
CUC |
CCA |
CUG |
CCC |
UUU |
G |
|
(RNA) |
-CAA |
AGG |
GCA |
GUG |
GGA |
GAA |
UAA |
AUU |
U |
|
SERPINB4-2 |
(RNA) |
-AGA |
UUU |
GUA |
UGU |
AUU |
CUU |
GUU |
UGG |
C |
|
(RNA) |
-GCC |
AAA |
CAA |
GAA |
UAC |
AUA |
CAA |
AUC |
U |
|
SERPINB4-3 |
(RNA) |
-AUC |
GAC |
ACA |
UGU |
CUC |
UCU |
CAU |
AUU |
C |
|
(RNA) |
-GAA |
UAG |
GAG |
AGA |
GAC |
AUG |
UGU |
CGA |
U |