Abstract
Free-grazing ducks play a major role in the rural economy of Eastern Asia in the form of egg and meat production. In Thailand, the geographical location, tropical climate conditions and wetland areas of the country are suitable for their husbandry. These environmental factors also favor growth, multiplication, development, survival, and spread of duck parasites. In this study, a total of 90 free-grazing ducks from northern, central, and northeastern regions of Thailand were examined for intestinal helminth parasites, with special emphasis on zoonotic echinostomes. Of these, 51 (56.7%) were infected by one or more species of zoonotic echinostomes, Echinostoma revolutum, Echinoparyphium recurvatum, and Hypoderaeum conoideum. Echinostomes found were identified using morphological criteria when possible. ITS2 sequences were used to identify juvenile and incomplete worms. The prevalence of infection was relatively high in each region, namely, north, central, and northeast region was 63.2%, 54.5%, and 55.3%, respectively. The intensity of infection ranged up to 49 worms/infected duck. Free-grazing ducks clearly play an important role in the life cycle maintenance, spread, and transmission of these medically important echinostomes in Thailand.
-
Key words: Echinostoma revolutum, Hypoderaeum conoideum, Echinoparyphium recurvatum, echinostome, free-grazing duck, prevalence, Thailand
INTRODUCTION
Echinostomes comprise a group of at least 60 species of foodborne and zoonotic intestinal trematodes, and are endemic worldwide [
1]. Human echinostomiasis has been reported, especially in Southeast Asia, caused by at least 20 species belonging to 8 genera, namely
Echinostoma,
Echinochasmus,
Acanthoparyphium,
Artyfechinostomum,
Episthmium,
Himasthla,
Hypoderaeum, and
Isthmiophora [
2,
3]. Asian countries from which human cases have been reported include India, the Philippines, China, Taiwan, Indonesia, Malaysia, the Republic of Korea, Japan, Cambodia, Lao PDR, and Thailand [
4-
7]. Seven species, i.e.,
Echinostoma revolutum,
Echinostoma ilocanum,
Echinostoma malayanum (syn.
Artyfechinostomum malayanum),
Echinochasmus japonicus,
Episthmium caninum,
Artyfechinostomum sp., and
Hypoderaeum conoideum have been reported as medically important species in Thailand [
8,
9] and
Echinoparyphium recurvatum has been reported to infect man in Taiwan, Indonesia, and Egypt [
10]. In 1969, more than 50% of northern Thai residents were infected with echinostomes [
11], but the prevalence had decreased to 0.7% by 1998 [
12]. In addition to Thailand, there are also recent studies reporting the incidence of human echinostomiasis in other areas of Greater Mekong Subregion; 1.1% in riparian villages along the Mekong River in Khammouane Province, Lao PDR [
13]; between 7.5-22.4% among schoolchildren in Pursat Province, Cambodia (eggs found were presumed to be those of
E. revolutum) [
14]; and a range of 0.7-1.8% in Oddar Meanchey Province, Cambodia, where the adult
E. ilocanum were recovered from humans [
15].
These medically important echinostomes infect a broad range of definitive hosts among wild, domestic, and peridomestic animals, e.g., cats, dogs, pigs, rodents, aquatic birds, chickens, and ducks [
16]. Infection occurs by ingestion of aquatic second intermediate hosts, i.e., freshwater snails, bivalves, fish, and tadpoles. Among the snail genera, those which have been reported as the first intermediate hosts of echinostomes in Thailand are
Indoplanorbis sp.,
Gyraulus sp.,
Lymnaea spp.,
Pilas sp.,
Viviparus sp., and
Filopaludina sp. [
17,
18]. The morbidity of echinostome infections in humans may be not too severe, but is dependent on the number of worms present [
5,
16,
19]. However, heavy infections with echinostomes, especially
E. revolutum, may cause emaciation and catarrhal enteritis, and death may occur in young and non-healthy animals, including ducks [
20]. Such birds and poultry act as major reservoirs of infection in both agricultural and natural settings. High prevalences of infection with zoonotic echinostomes
E. revolutum,
E. recurvatum, and
H. conoideum have been reported in domestic ducks in Bangladesh [
20], and
Echinostoma cinetorchis and
H. conoideum in Vietnam [
21].
Nowadays, free-grazing domestic ducks are common in Southeast Asia particularly in Thailand. Rice paddy fields used for double-crop rice provide excellent year-round foraging for them. Ducks are released on paddy fields after harvest and feed on leftover rice grains, wild rice, insects, and aquatic animals including many intermediate hosts of echinostomes such as those listed above. Rotation of free-grazing ducks between rice paddy fields may play an important role in the life cycle maintenance, spread, and transmission of foodborne zoonotic parasites, particularly echinostomes. Rotation occurs at frequencies depending on the availability of food, but usually every few weeks.
Gastrointestinal parasites of poultry are common worldwide [
20-
23]. Very little is known about echinostome infections in free-grazing ducks in Thailand. Our survey provided significant information on this topic, aiding management and control programs for these zoonotic echinostomes.
MATERIALS AND METHODS
Sampling method
We purchased the intestines of ducks from local slaughterhouses in central, north, and northeast regions of Thailand. Those ducks were from flocks grazing rice paddy fields used for double-crop rice production. A total of 90 samples, consisting of 19 from 3 areas in northern Thailand, 33 from 4 areas in central Thailand, and 38 from 5 areas in northeastern Thailand were examined between May 2011 and February 2012 (
Table 1;
Fig. 1).
The intestines of ducks were opened and any visible trematodes were picked out and placed in a separate Petri-dish containing normal saline. After that, the intestines were washed to find any remaining worms. The worms recovered from an individual duck were pooled in a Petri-dish and washed several time using saline. For identification purposes, each adult worm was flattened between 2 glass slides, held apart by a small piece of filter paper, and then examined under a light microscope. Species identification was based on the testis shape, development of the circumoral disc, and number of collar spines [
24]. Any worms that could not be identified in this way were subjected to molecular analysis. Ten worms of each morphologically identifiable species (
E. revolutum and
H. conoideum) were randomly selected and fixed in 10% formalin and subsequently washed in 70% ethanol several times to remove formalin, stained with carmine for 1 day, and destained with 1% HCl in distilled water until the flukes appeared pink in color. After that the flukes were dehydrated in 70%, 80%, 95%, and 100% ethanol, consecutively for 2 hr each. Finally, the dehydrated flukes were mounted with permount solution on glass slides and identified by morphological features following the published keys [
8,
24].
Genomic DNA (gDNA) was individually extracted from each worm using the DNA extraction kit (QIAGEN, Hilden, Germany) following the manufacturer's protocol. The ITS2 region was amplified using primers and PCR conditions following our previous report [
25]. Amplicons were sequenced using the dideoxynucleotide chain termination method using Dye Primer and Dye Terminator Cycle sequencing kits (Applied Biosystem Inc., Foster City, California, USA) and an ABI DNA sequencer 373A. The ITS2 sequences obtained in this study were aligned with the sequences of known species retrieved from GenBank database using BioEdit program version 5.0.6.
RESULTS
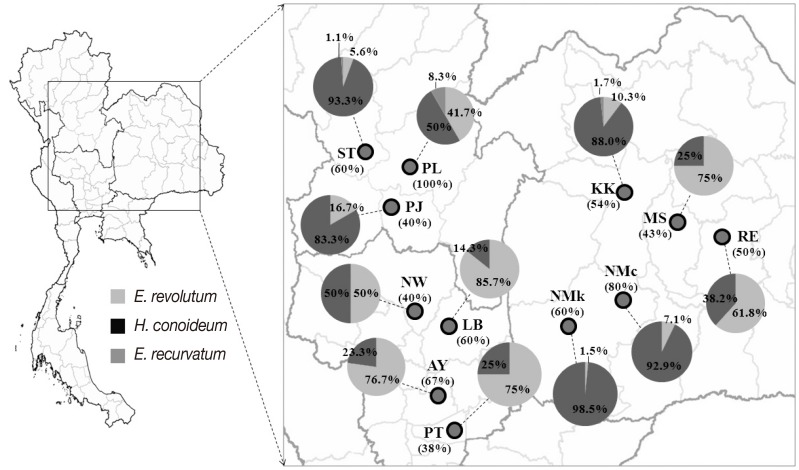
Of the 90 free-grazing ducks examined, 51 (56.7%) were infected by 1 or more species of the 3 zoonotic echinostomes,
E. revolutum,
E. recurvatum, and
H. conoideum. The prevalence of infection in each geographical area is relatively high, ranging between 38% and 100% (
Fig. 1). Prevalences in general did not differ greatly between regions, i.e., 63.2% in north (12/19), 54.5% in central (18/33), and 55.3% in northeast (21/38). In terms of the prevalences of each echinostome species,
E. revolutum was the most common worm in the central region and Roi Et and Maha Sarakham Provinces of the northeast region. Elsewhere,
H. conoideum was the most common species (
Table 1;
Fig. 1). Eight unidentified worms could be confirmed by ITS2 sequences as being 2
H. conoideum and 6
E. recurvatum (included in the data in
Table 1 and
Fig. 1).
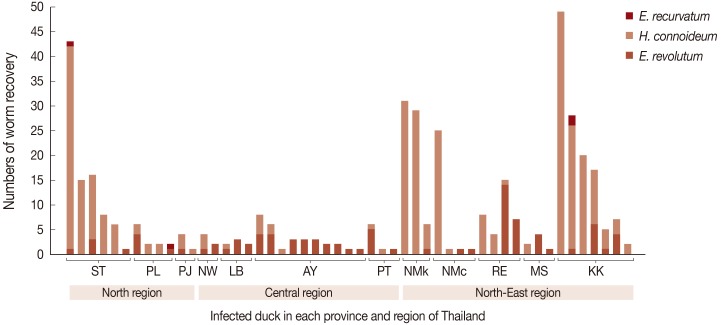
The maximum number of worms in an infected duck was 49.
H. conoideum was the highest in number (
Fig. 2). Of the 51 infected ducks, 17 (33.3%) were infected by
E. revolutum alone, 18 (35.3%) by
H. conoideum alone, 13 ducks (25.5%) had mixed infections with
E. revolutum and
H. conoideum, 1 (2.0%) had mixed infections with
E. revolutum and
E. recurvatum, and 2 (3.9%) harbored all 3 echinostome species (
Fig. 2).
DISCUSSION
Farmers in Southeast Asia use flocks of free-grazing ducks to help control invasive golden apple snails,
Pomacea canaliculata, originally native to South America, which can cause extensive damage to rice paddies. The golden apple snails, other pond snails, e.g.,
Lymnaea sp.,
Indoplanorbis sp.,
Filopaludina sp., and even tadpoles, and small freshwater fish, all of which can act as the second intermediate hosts of echinostomes, are also eaten by those ducks. Consequently, high rates of echinostome infection are found in free-grazing ducks in Thailand, as revealed in this study and previous reports from other countries, e.g., Bangladesh [
20,
26], Korea [
23], and Vietnam [
21]. However, the presence of echinostomes in free-grazing ducks is not entirely a bad thing.
E. revolutum has proven to be an effective biological control agent of the highly pathogenic fluke species,
Fasciola gigantica, when the 2 trematodes share the same snail intermediate host,
Lymnaea rubiginosa [
27].
The infectious prevalence of each echinostome species varied geographically and may reflect the differences in grazing system used by particular farmers. However, many factors may influence this phenomenon, previous work indicates that 2 sibling species of
E. revolutum from Thailand and Laos probably exist [
28,
29]. Temporal factors cannot be excluded. Reports exist noting that high prevalences of helminths in poultry are usually found in winter and rainy seasons. This may be related to persistence of parasites in intermediate hosts in some seasons, and abundance of intermediate hosts in others [
19,
26]. Moreover, the prevalence of infection was probably correlated to the sex and age of ducks. It has been reported previously that female ducks and those over a year old have higher prevalences of echinostomes infections than male ducks and ducks under 1 year of age, respectively [
20]. However, in our study, all ducks were females (for laying eggs) and above 1 year in age.
Ducks are usually rotated among several rice paddies within restricted geographical areas (within a province). This rotation within a province is controlled by local laws and the local department of livestock development. The frequency of translocations is dependent on the food available at each site (rice grains, snails, and other small aquatic animals). Normally, the farmer will rotate the duck flock by vehicle every few weeks. The flocks are allowed to feed in rice paddies after the harvesting season but will be removed before the planting season. The high prevalence of these zoonotic echinostomes in free-grazing ducks suggests their role as reservoir hosts in Thailand. Therefore, the owners of ducks must be educated on the risks with this practice as a constituent of any incorporated foodborne zoonotic trematode prevention and control program.
Thammasat UniversityTRF-CHE-MSUMRG5480009
Thailand Research FundRTA5580004
ACKNOWLEDGMENTS
This study was supported by Faculty of Medicine, Thammasat University grant to C. Tantrawatpan, TRF-CHE-MSU grant to W. Saijuntha (grant no. MRG5480009) and was also supported in part by TRF Senior Research Scholar Grant, Thailand Research Fund grant no. RTA5580004. We wish to thank the Institute Research and Development, Department of Public Health, Faculty of Science and Technology, Phetchabun Rajabhat University, Thailand.
References
- 1. Sorensen RE, Curtis J, Minchella DJ. Intraspecific variation in the rDNA its loci of 37-collar-spined echinostomes from North America: implications for sequence-based diagnoses and phylogenetics. J Parasitol 1998;84:992-997.
- 2. Chai JY, Murrell KD, Lymbery AJ. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol 2005;35:1233-1254.
- 3. Chai JY, Shin EH, Lee SH, Rim HJ. Foodborne intestinal flukes in Southeast Asia. Korean J Parasitol 2009;47(suppl):S69-S102.
- 4. Miliotis MD, Bier JW. International Handbook of Foodborne Pathogens. New York. CRC Press; 2003.
- 5. Graczyk TK, Fried B. Echinostomiasis: a common but forgotten food-borne disease. Am J Trop Med Hyg 1998;58:501-504.
- 6. Fried B, Graczyk TK, Tamang L. Food-borne intestinal trematodiases in humans. Parasitol Res 2004;93:159-170.
- 7. Grover M, Dutta R, Kumar R, Aneja S, Mehta G. Echinostoma ilocanum infection. Indian Pediatr 1998;35:549-552.
- 8. Radomyos P, Krudsood S, Wilairatana P, Looareesuwan S. Atlas of Medical Parasitology. Bangkok, Thailand. Pappim Co. Ltd; 2003, (in Thai).
- 9. Joe LK, Nasemary S, Impand P. Five echinostome species from Thailand. Southeast Asian J Trop Med Public Health 1973;4:96-101.
- 10. Beaver PC, Jung RC, Cupp EW. Clinical Parasitology. 9th ed. Philadelphia, USA. Lea & Febiger; 1984, p 460.
- 11. Sornmani S. Echinostomiasis in Thailand: a review. Southeast Asian J Trop Med Public Health 1969;1:171-175.
- 12. Radomyos B, Wongsaroj T, Wilairatana P, Radomyos P, Praevanich R, Meesomboon V, Jongsuksuntikul P. Opisthorchiasis and intestinal fluke infections in northern Thailand. Southeast Asian J Trop Med Public Health 1998;29:123-127.
- 13. Chai JY, Sohn WM, Yong TS, Eom KS, Min DY, Hoang EH, Phammasack B, Insisiengmay B, Rim HJ. Echinostome flukes recovered from humans in Khammouane Province, Lao PDR. Korean J Parasitol 2012;50:269-272.
- 14. Sohn WM, Chai JY, Yong TS, Eom KS, Yoon CH, Sinuon M, Socheat D, Lee SH. Echinostoma revolutum infection in children, Pursat Province, Cambodia. Emerg Infect Dis 2011;17:117-119.
- 15. Sohn WM, Kim HJ, Yong TS, Eom KS, Jeong HG, Kim JK, Kang AR, Kim MR, Park JM, Ji SH, Sinuon M, Socheat D, Chai JY. Echinostoma ilocanum infection in Oddar Meanchey Province, Cambodia. Korean J Parasitol 2011;49:187-190.
- 16. Huffman JE, Fried B. Echinostoma and echinostomiasis. Adv Parasitol 1990;29:215-269.
- 17. WHO. Control of food-borne trematode infections. World Health Organ Tech Rep Ser 1995;849:1-157.
- 18. Pariyanonda S, Tesana S. Edible mollusk, the intermediate host of helminthes in Khon Kaen Province, Thailand. Srinagarind Hosp Med J 1990;5:159-172.
- 19. Chai JY, Hong ST, Lee SH, Lee GC, Min YI. A case of echinostomiasis with ulcerative lesions in the duodenum. Korean J Parasitol 1994;32:201-204.
- 20. Yousuf MA, Das PM, Anisuzzaman , Banowary B. Gastro-intestinal helminths of ducks: Some epidemiologic and pathologic aspects. J Bangladesh Agr Univ 2009;7:91-97.
- 21. Anh NT, Madsen H, Dalsgaard A, Phuong NT, Thanh DT, Murrell KD. Poultry as reservoir hosts for fishborne zoonotic trematodes in Vietnamese fish farms. Vet Parasitol 2010;169:391-394.
- 22. Saijuntha W, Sithithaworn P, Andrews RH. Genetic differentiation of Echinostoma revolutum and Hypodereaum conoideum from domestic ducks in Thailand by multilocus enzyme electrophoresis. J Helminthol 2010;84:143-148.
- 23. Eom KS, Rim HJ, Jang DH. A study on the parasitic helminths of domestic duck (Anas platyrhynchos var. domestica Linnaeus) in Korea. Korean J Parasitol 1984;22:215-221.
- 24. Kostadinova A. Family Echinostomatidae Looss, 1899.. In Jones A, Bray RA, Gibson DI eds, Keys to the Trematoda. Vol. 2:London, UK. CABI Publishing and the Natural History Museum; 2005, pp 9-64.
- 25. Tantrawatpan C, Saijuntha W, Sithithaworn P, Andrews RH, Petney TN. Genetic differentiation of Artyfechinostomum malayanum and A. sufrartyfex (Trematoda: Echinostomatidae) based on internal transcribed spacer sequences. Parasitol Res 2013;112:437-441.
- 26. Anisuzzaman , Alim MA, Rahman MH, Mondal MMH. Helminth parasites in indigenous ducks: Seasonal dynamics and effects on production performance. J Bangladesh Agr Univ 2005;3:283-290.
- 27. Suhardono , Roberts JA, Copeman DB. Biological control of Fasciola gigantica with Echinostoma revolutum. Vet Parasitol 2006;140:166-170.
- 28. Saijuntha W, Sithithaworn P, Duenngai K, Kiatsopit N, Andrews RH, Petney TN. Genetic variation and relationships of four species of medically important echinostomes (Trematoda: Echinostomatidae) in South-East Asia. Infect Genet Evol 2011;11:375-381.
- 29. Saijuntha W, Tantrawatpan C, Sithithaworn P, Andrews RH, Petney TN. Spatial and temporal genetic variation of Echinostoma revolutum (Trematoda: Echinostomatidae) from Thailand and the Lao PDR. Acta Trop 2011;118:105-109.
Fig. 1Map showing the prevalence of infection in free-grazing ducks (in parentheses under the area code) and infection rates of each echinostome species (indicated in the pie-charts) in each sampled area (see more details in
Table 1).
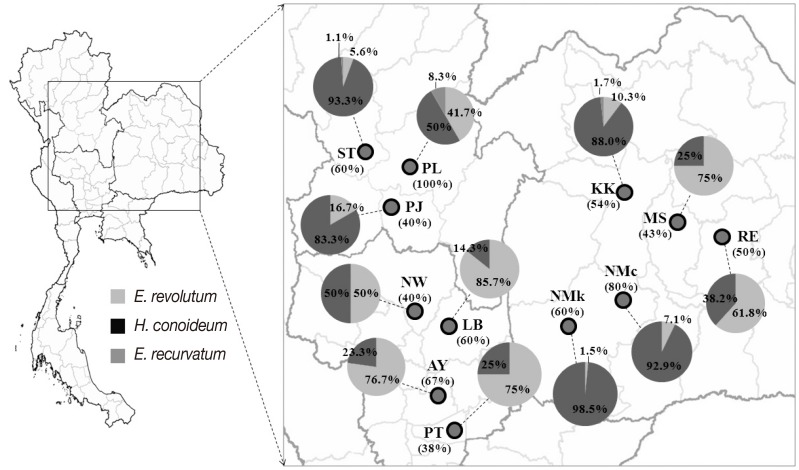
Fig. 2Numbers of adult worms recovered per infected duck.
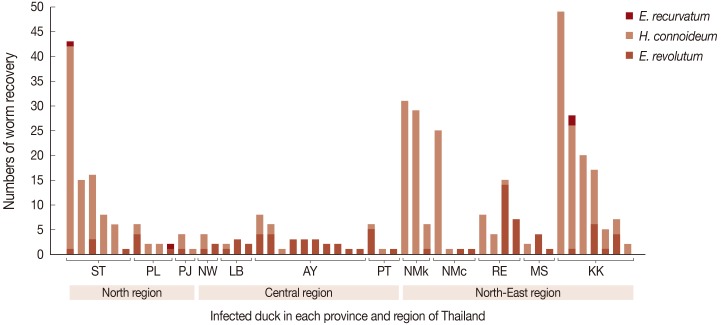
Table 1.Survey localities for echinostomes infections in free-grazing ducks
Table 1.
|
Province (code) |
District |
Region |
Na
|
No. infected |
No. of worms recovered
|
|
E. revolutum
|
H. conoideum
|
E. recurvatum
|
Total |
|
Sukhothai (ST) |
Kong Krailat |
North |
10 |
6 |
5 (5.6%) |
83 (93.3%) |
1 (1.1%) |
89 |
|
Phitsanulok (PL) |
Mueang |
North |
4 |
4 |
5 (41.7%) |
6 (50.0%) |
1 (8.3%) |
12 |
|
Phichit (PJ) |
Bueng Na Rang |
North |
5 |
2 |
1 (16.7%) |
5 (83.3%) |
0 |
6 |
|
Nakhon Sawan (NW) |
Takhli |
Central |
5 |
2 |
3 (50.0%) |
3 (50.0%) |
0 |
6 |
|
Lop Buri (LB) |
Ban Mi |
Central |
5 |
3 |
6 (85.7%) |
1 (14.3%) |
0 |
7 |
|
Ayutthaya (AY) |
Bang Ban |
Central |
15 |
10 |
23 (76.7%) |
7 (23.3%) |
0 |
30 |
|
Pathum Thani (PT) |
Khlong Laung |
Central |
8 |
3 |
6 (75.0%) |
2 (25.0%) |
0 |
8 |
|
Nakhon Ratchasima (NMk) |
Kham Thale Sor |
North-East |
5 |
3 |
1 (1.5%) |
65 (98.5%) |
0 |
67 |
|
Nakhon Ratchasima (NMc) |
Chum Phuang |
North-East |
5 |
4 |
2 (7.1%) |
26 (92.9%) |
0 |
28 |
|
Roi Et (RE) |
Changhan |
North-East |
8 |
4 |
21 (61.8%) |
13 (38.2%) |
0 |
34 |
|
Maha Sarakham (MS) |
Mueang |
North-East |
7 |
3 |
6 (75.0%) |
2 (25.0%) |
0 |
8 |
|
Khon Kaen (KK) |
Mueang |
North-East |
13 |
7 |
12 (10.3%) |
103 (88.0%) |
2 (1.7%) |
117 |