Abstract
Several reports on taeniasis and cysticercosis in Vietnam show that they are distributed in over 50 of 63 provinces. In some endemic areas, the prevalence of taeniasis was 0.2-12.0% and that of cysticercosis was 1.0-7.2%. The major symptoms of taeniasis included fidgeted anus, proglottids moving out of the anus, and proglottids in the feces. Clinical manifestations of cysticercosis in humans included subcutaneous nodules, epileptic seizures, severe headach, impaired vision, and memory loss. The species identification of Taenia in Vietnam included Taenia asiatica, Taenia saginata, and Taenia solium based on combined morphology and molecular methods. Only T. solium caused cysticercosis in humans. Praziquantel was chosen for treatment of taeniasis and albendazole for treatment of cysticercosis. The infection rate of cysticercus cellulosae in pigs was 0.04% at Hanoi slaughterhouses, 0.03-0.31% at provincial slaughterhouses in the north, and 0.9% in provincial slaughterhouses in the southern region of Vietnam. The infection rate of cysticercus bovis in cattle was 0.03-2.17% at Hanoi slaughterhouses. Risk factors investigated with regard to transmission of Taenia suggested that consumption of raw meat (eating raw meat 4.5-74.3%), inadequate or absent meat inspection and control, poor sanitation in some endemic areas, and use of untreated human waste as a fertilizer for crops may play important roles in Vietnam, although this remains to be validated.
-
Key words: Taeniasis, cysticercosis, Vietnam
INTRODUCTION
Taeniasis and cysticercosis in humans are meat-borne parasitoses. The adult stage of the worm (
Taenia saginata,
Taenia solium, and
Taenia asiatica) develops only in the intestine of the human host. Human cysticercosis is a tissue-infecting parasitosis caused by ingestion of
T. solium eggs. The cysticerci of
T. saginata occur only in beef, and those of
T. asiatica in pig visceral organs [
1,
2]. The cysticerci of
T. solium occur primarily in pork, but they also occur in humans. Therefore, humans can have taeniasis and cysticercosis (including neurocysticercosis) [
1,
2].
T. asiatica is commonly known as Asian
Taenia or Asian tapeworm and is a parasitic tapeworm of humans and pigs [
3].
It has been estimated that hundreds of millions of persons worldwide are infected with
T. solium, the most serious tapeworm species to humans [
4,
5,
6]. Human taeniasis and cysticercosis are diseases that occur in areas where people eat raw or undercooked meat, and traditional pig/cattle husbandry is practiced [
2]. They are endemic in Africa, South and Central America, Brazil, Mexico, China, India, Myanmar, Malaysia, Korea, Indonesia, Philippines, and Southeast Asia, including Vietnam [
2,
4].
HUMAN TAENIASIS IN VIETNAM
Human taeniasis was found from more than 50 provinces in Vietnam. The prevalence of taeniasis was 0.5-12% in the north of the country, 0.2-2.8% in the middle, 0.3-1.5% in the south, and 3.8-10.1% in the highlands [
7,
8]. The prevalence of taeniasis in plain areas was 0.5-12.0% and in mountainous areas 2.0-10.4% [
7,
8].
The infection rate of taeniasis in males was higher than that in females in Vietnam (68.2% compared to 31.8%).
Taenia eggs were found in 20.0-22.5% of taeniasis patients by stool examinations. Soil samples in endemic areas were examined to find
Taenia eggs, and the results showed that the positive rate of Taenia eggs was 2.0-25.0% [
8]. Some patients were infected with more than 2 adult worms per person (
Fig. 1).
Somers et al. [
9] reported that
T. asiatica was the most common species (55.4%) in Vietnam over
T. saginata (38.5%) and
T. solium (6.2%) out of a total of 65
Taenia samples collected from patients in a referral hospital in Hanoi, north Vietnam (species identification by morphological and molecular methods using PCR-RFLP of a mitochondrial 12S rDNA [
9]. Trieu [
10] confirmed 69
Taenia samples,
T. asiatica 53.0%,
T. saginata 34.8%, and
T. solium 23.2% using the multiplex PCR method [
10]. Vien et al. [
11] identified 65
Taenia samples which consisted of
T. asiatica 58.5%,
T. saginata 41.5%, and
T. solium 0% using the multiplex PCR method.
HUMAN CYSTICERCOSIS
Human cysticercosis is distributed in more than 50 provinces, especially in the north of the country such as Bac Ninh Province, Thai Binh Province, and Thanh Hoa Province, where the local people have a habit of eating raw pork. The feeding pigs are roaming around the house, and the hygienic conditions are low. The prevalence of cysticercosis was 1.0-7.2% in the north and 4.3% in the southern regions of the country [
7,
8].
There are hundreds of cysticercosis patients treated in the clinic of National Institute of Malariology, Parasitology and Entomology (NIMPE) every year. For example, during 1996-1997, 633 were cysticercosis (15.8%) patients out of 4,017 in-patients admitted to the NIMPE Clinic, and in 2010, 229 cysticercosis patients were diagnosed out of 1,524 parasitic in-patients (15.0%) [
8,
12].
PORCINE AND BOVINE CYSTICERCOSIS
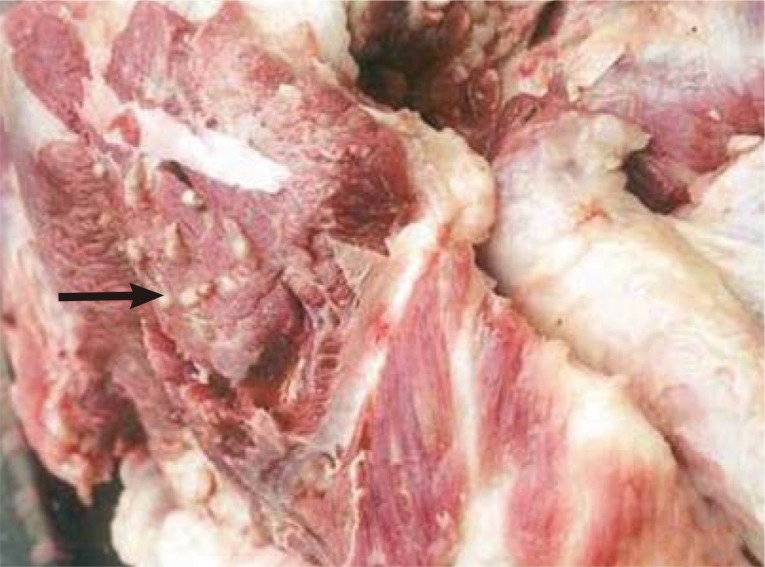
The prevalence of porcine cysticercosis was 0.04% (835/2,237,000) at Hanoi slaughterhouses, 0.06% (109/172,087) and 0.03-0.31% (out of 198,887) at provincial slaughterhouses in the north; and 0.9% (8/891) in provincial slaughterhouses in the southern regions of Vietnam [
8,
10] (
Fig. 2).
The organs affected in porcine cysticercosis were the eye (31.0%), jaw (14.2%), throat (12.2%), tongue (9.3%), shoulder (8.3%), back (6.0%), neck (6.3%), chest (5.3%), rump (4.6%), and diaphragm (2.3%) with 1,067 cysts per 10 kg pork [
8].
The prevalence of cysticercus bovis in cattle was 1.6% in general, 0.03% (39/144,390) at Hanoi slaughterhouses, 0.5-1.4% in the north, 1.9-2.2% in the middle, and 1.6-1.8% in the south of the country [
8,
12].
RISK FACTORS FOR HUMAN TAENIASIS AND CYSTICERCOSIS
Some surveys investigating risk factors for human taeniasis and cysticercosis in Vietnam have indicated that important factors include consuming raw meat and unwashed vegetables, allowing pigs to roam, poor sanitation, lack of hygienic latrines, use of human feces for a fertilizer, and no or inadequate meat inspection [
10]. The habit of eating raw meat is very common in Vietnam. The rate of eating raw pork or beef was 4.5-47.5% in the north, 74.3% in the middle, and 4.5-23.0% in the south of the country [
8].
The latrine present was 72.8-99.4% in the north, 62.2-96.3% in the middle, and 70.4-88.7% in the south, but defecation outdoors was 12.2-15.4% in the north, 20.2-25.3% in the middle, and 11.7-17.6% in the south. Use of night-soil fertilizer was 5.0-18.4% in the north, 4.2-16.3% in the middle, and 6.3-7.5% in the south [
8].
SYMPTOMS AND DIAGNOSIS OF TAENIASIS AND CYSTICERCOSIS
The major symptoms of human taeniasis included fidgeted anus 63.7-80.2%, proglottids moving to the anus or with the feces 82.9-92.1%, abdominal pain 59.7-62.1%, digestive disorder 45.2-50.0%, sleeplessness 35.2-39.5%, hypotension 10.4-12.1%, positive
Taenia eggs in stool 18.1-22.5%, increased eosinophils 76.1-81.1%, and ELISA positives with
Taenia antigen 72.2-76.7% [
8,
10,
12].
The major symptoms of human cysticercosis included headache 65.9-90.4%, epilepsy 56.4-67.5%, quickened muscle 35.3-48.9%, losing memories 42.4-46.9%, sleeplessness 40.3-46.4%, hearing disorders 36.4-42.4%, nausea/vomiting 39.2-40.8%, paralysis 28.4-30.8%, feeling disorder 18.2-20.1%, biopsy positives in subcutaneous tissues 92.2-95.4%, increased eosinophils 83.4-84.5%, positive ELISA with
Taenia antigen 85.1-86.6%, and positive MRI/CT scan with live cysts in the brain 90.1-92.2% [
8,
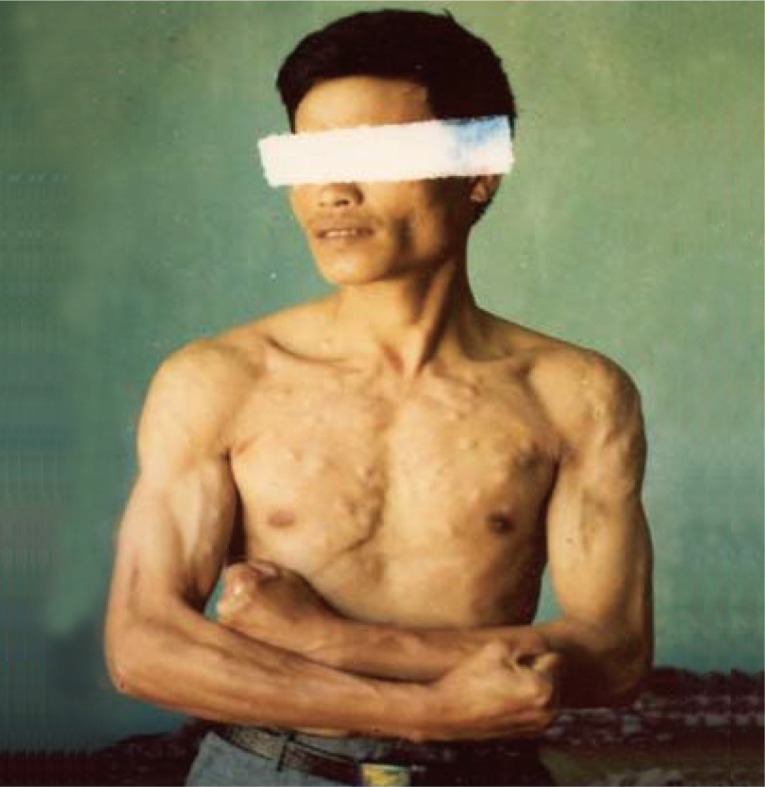
10]. Subcutaneous cysts were found in back-chest 36.6%, hands 28.7%, head-face-neck 18.2%, and legs 17.4%. The highest number of subcutaneous nodules was 300 cysts in a patient (
Fig. 3) [
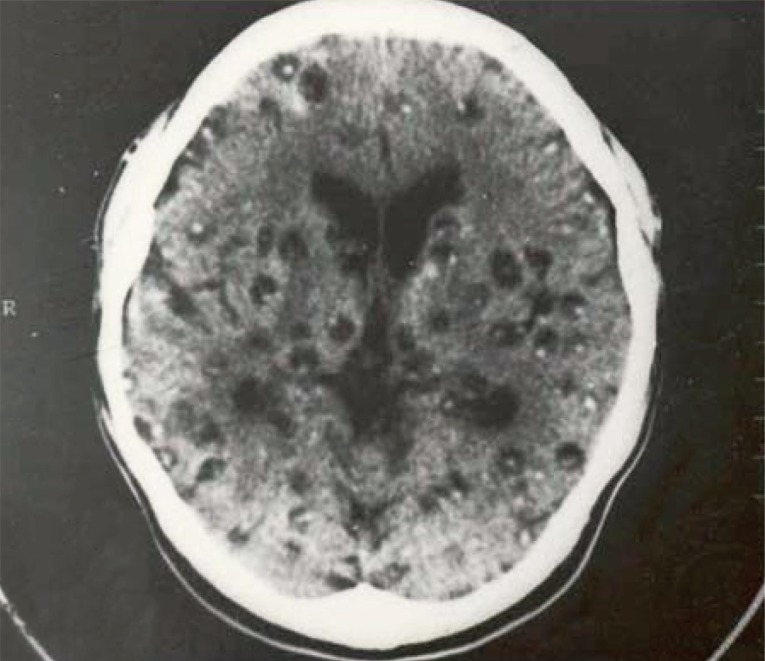
13]. Regarding the number of cysticercus cellulosae cysts in the brain, patients with <20 cysts were 53.3%, and those with ≥20 cysts was 46.7%. The highest number of cysticerci in the brain was 300 (
Fig. 4) [
13].
In 100 neurocysticercosis patients, the form of cysts included vesicular nodule 83%; colloidal vesicle 100%; granular nodule 81%, calcified nodule 96%, mixture of 4 forms 78%; and colloidal vesicle only 11%. There were 50% patients that showed spread-stimulated waves in electro-encephalography, 27% patients that showed local stimulated waves, and 9% patients with unusual electro-encephalography with theta waves [
8].
TREATMENT OF TAENIASIS AND CYSTICERCOSIS
Adult worm (taeniasis) was treated with niclosamide single dose 2 g (cure rate, 66-90%) or with praziquantel 18 mg-20 mg/kg body, single dose (cure rate, 97.8-100%) [
8,
12].
Cysticercosis was treated with albendazole 15 mg/kg body/day×20 consecutive days for 3 courses (in the first day for the first course using praziquantel single dose 18 mg-20 mg/kg body). The result showed the clearance of subcutaneous nodules, 91.5-96.3%; clearance of live cysts in brain, 50-57%; cure rate of epilepsy, 70.4-96.6%; cure rate of headache, 78.2-97.6% [
8,
12].
Treatment of cysticercosis was done with praziquantel 30 mg/kg/day×20 consecutive days for 3 courses. The results showed that, the clearance of subcutaneous nodule was 85.4-90.0%, clearance of live cysts in brain was 23.5-48.7%, cure rate of epilepsy was 68.4-77.7%, cure rate of headaches was 87.9-92.3% [
8].
SPECIES IDENTIFICATION OF TAENIA AND CYSTICERCUS
The multiplex PCR to identify
Taenia species in Vietnam [
14] revealed that there are 3 species including
T. asiatica (42.0-58.5%) which has a specific band of 706 bp,
T. saginata (34.8-41.5%) which has a 629 bp band, and
T. solium (6.2-23.2%) which has a 474 bp band. The subcutaneous nodules of cysticercosis from patients in 30 provinces in the north of Vietnam, all were identified as
T. solium metacestode by the multiplex PCR [
8,
6,
11,
12].
Using sequences of a portion of 652 bp, the mitochondrial-encoded cob gene was amplified by PCR [
15] of different human
Taenia sp. samples with different forms of adults collected from 40 provinces in the north, middle, and south of the country. The nucleotide and amino acid sequences of the Vietnamese
Taenia sp. samples were aligned with the known corresponding sequences of
T. asiatica (geographical origin, Taiwan, with 98-99% homology),
T. saginata (geographical origin, China, with 99-100% homology),
T. solium (geographical origin, China and global strains, with 99-100% homology); and other related Taenia species (GenBank and published data) using special programs. There is very high nucleotide and amino acid similarities between the Vietnamese isolates (human), and cow and pig tapeworm matacestodes of different origin, respectively. Phylogenetic analysis indicated that the Vietnamese
T. solium is clustered with the Asian
T. solium species, while the Vietnamese
T. asiatica and
T. saginata were clustered with Taiwanese and Chinese isolates, respectively [
8,
12]. The first
T. asiatica infection in Vietnam was reported by De et al. [
16] in 2001. The phylogenetic relationships of the Vietnamese
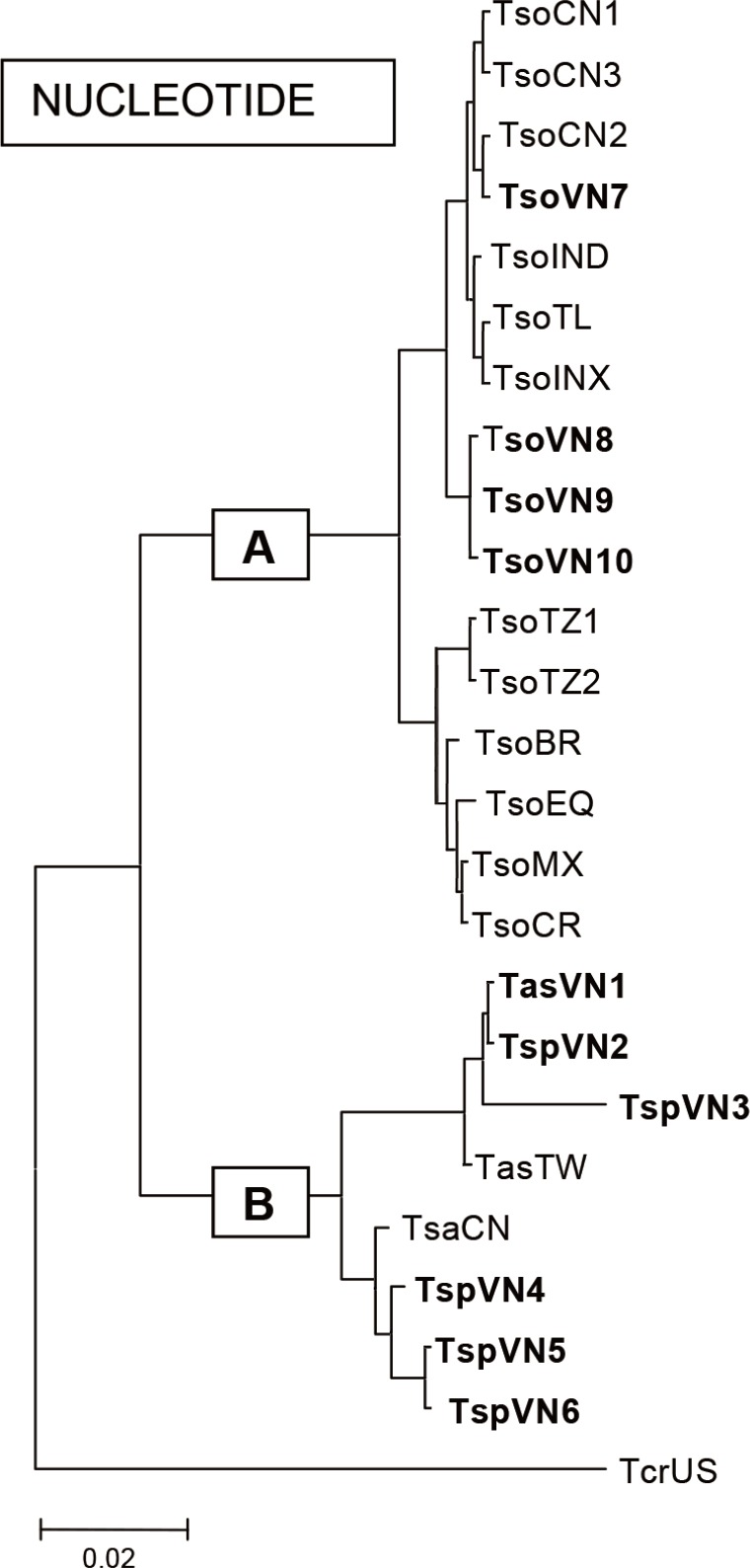
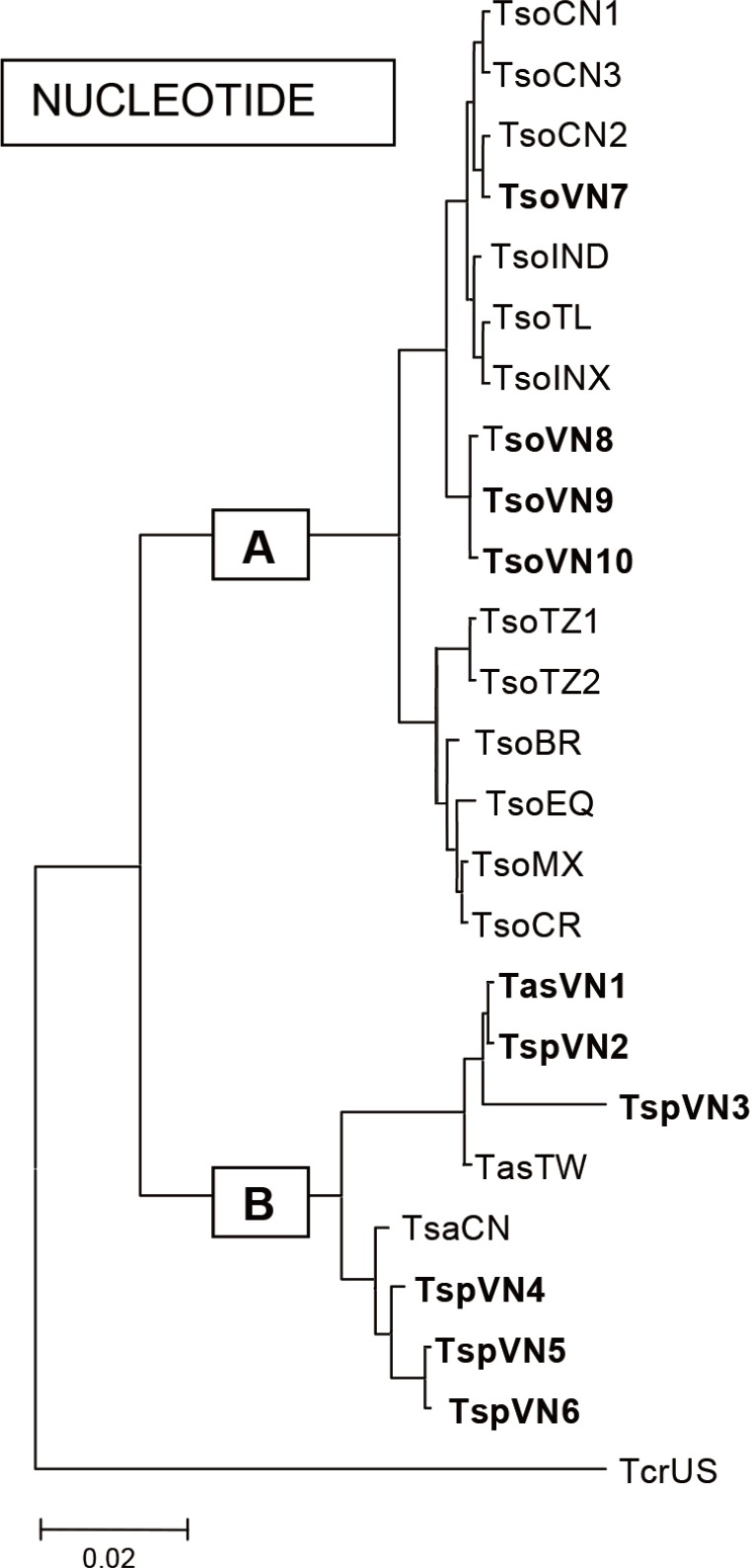
Taenia and other representative species in the world were analyzed comparing their nucleotides. The results showed that group A included
T. solium and group B included
T. asiatica,
T. saginata, and others including
T. crassiceps (
Fig. 5).
The subcutaneous nodules of cysticercosis from patients in 30 provinces were identified as 100%
T. solium metacestodes by sequencing and homology of the nucleotides and amino acids compared with the Chinese origin of
T. solium with 99.1% and 100.0% homology, respectively [
8].
CONCLUSION
Taeniasis and cysticercosis are widely distributed in Vietnam and cause danger to health, especially neurocysticercosis. Species of Taenia in Vietnam includes T. asiatica, T. saginata, and T. solium, and only T. solium caused cysticercosis in humans. The big problem for control of these diseases is reducing the risk factors.
National Foundation for Science and Technology Development (NAFOSTED) in Vietnam106.12-2011.13
National Institute of Malariology, Parasitology and Entomology, Vietnam
Notes
-
We have no conflict of interest related to this study.
ACKNOWLEDGMENTS
The authors acknowledge the funds supported from the National Foundation for Science and Technology Development (NAFOSTED) in Vietnam (No. 106.12-2011.13 to Nguyen Van De), National Institute of Malariology, Parasitology and Entomology, Vietnam.
References
- 1. Fan PC, Chung WC. Taenia saginata asiatica: epidemiology, infection, immunological and molecular studies. J Microbiol Immunol Infect 1998;31:84-89.
- 2. Murrell KD. WHO/FAO/OIE guidelines for the surveillance, prevention and control of taeniosis/cysticercosis. Geneva, Switzerland. WHO; 2005, pp 75-80.
- 3. Eom KS, Rim HJ. Morphologic descriptions of Taenia asiatica sp. n. Korean J Parasitol 1993;31:1-6.
- 4. Ito A, Nakao M, Wandra T. Human taeniasis and cysticercosis in Asia. Lancet 2003;362:1918-1920.
- 5. Nakao M, Okamoto M, Sako Y, Yamasaki H, Nakaya K, Ito A. A phylogenetic hypothesis for the distribution of two genotypes of the pig tapeworm Taenia solium worldwide. Parasitology 2002;124:657-662.
- 6. Rajshekhar V, Joshi DD, Doanh NQ, De NV, Zhou X. Taenia solium taeniosis/cysticercosis in Asia: epidemiology, impact and issues. Acta Trop 2003;87:53-60.
- 7. De NV. Taenia and cysticercosis in Vietnam. Joint international Medicine Meeting. 29 November-1 December 2004; Bangkok Thailand.
- 8. De NV, Le TH. Taenia/cysticercosis and molecular application (textbook). Hanoi, Vietnam. Medical Publishing House; 2010, (in Vietnamese).
- 9. Somers R, Dorny P, Geysen D, Nguyen LA, Thach DC, Vercruysse J, Nguyen VK. Human tapeworms in north Vietnam. Trans R Soc Trop Med Hyg 2007;101:275-277.
- 10. Trieu HS. Study on genotype of pathogen, clinical, sub-clinical symptoms, treatment efficacy for taeniasis and cysticercosis patients in National Institute of Malariology, Parasitology and Entomology 2007-2010. 2012. PhD thesis (in Vietnamese).
- 11. Vien HV, Dao LD, Manh ND, Tan HV, Nguyen DH, Nhung VT. Identification of Taenia spp. and cysticercus sppecies using multiplex PCR. J Malaria Parasit Dis 2008;1:62-69. (in Vietnamese).
- 12. Willingham AL III, De NV, Doanh NQ, Cong LD, Dung TV, Dorny P, Cam PD, Dalsgaard A. Current status of cysticercosis in Vietnam. Southeast Asian J Trop Med Public Health 2003;34(suppl 1):35-50.
- 13. De NV, Le TH. Multiple palpable cysts. N Engl J Med 2013;368:2125.
- 14. Gonzalez LM, Montero E, Harrison L, Garate T. Differential diagnosis of Taenia saginata and Taenia solium infection by PCR. J Clin Microbiol 2000;38:737-744.
- 15. Le TH, Blair D, McManus DP. Mitochondrial genomes of parasitic flatworms. Trends Parasitol 2002;18:206-213.
- 16. De NV, Le TH, Doanh NQ, Nga NB, Cong LD. The first time report of the new species of Taenia in Vietnam. J Malaria Parasit Dis 2001;3:80-86. (in Vietnamese).
Fig. 1Adult Taenia tapeworms collected from a patient. Scale=2.5 cm.

Fig. 2Cysticercus cellulosae in pork in a market (reproduced from [
8]).
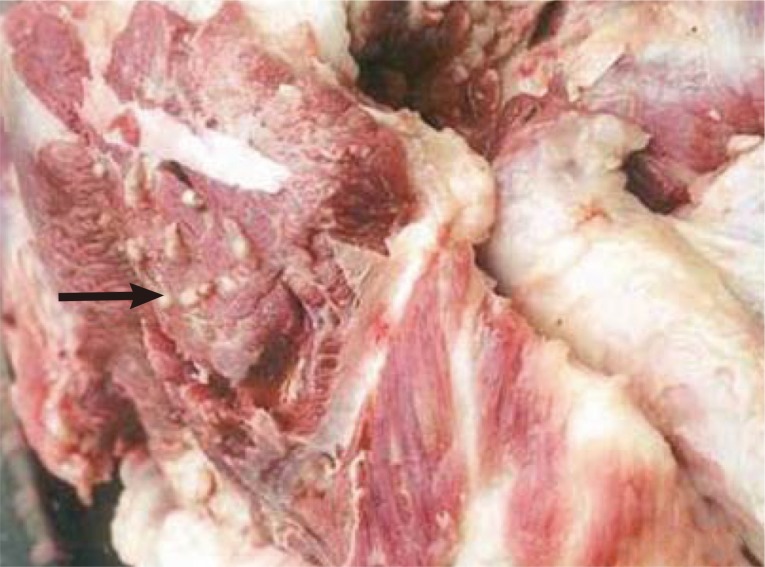
Fig. 3Subcutaneous nodules of a cysticercosis patient with about 300 recovered cysts in total (reproduced from [
12]).
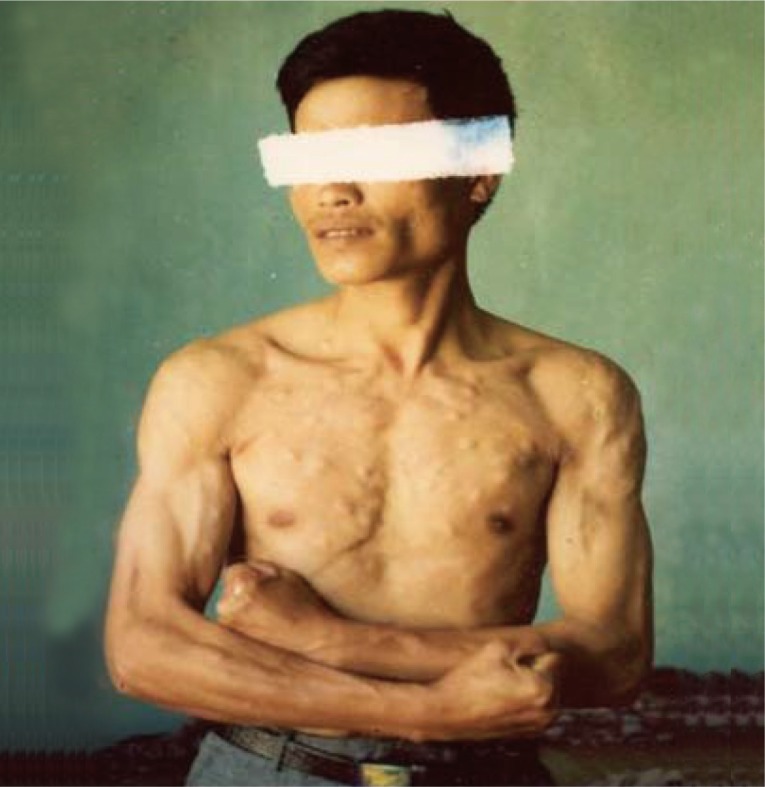
Fig. 4Cerebral cysts in a neurocysticercosis patient with about 300 recovered cysts in total (reproduced from [
12]).
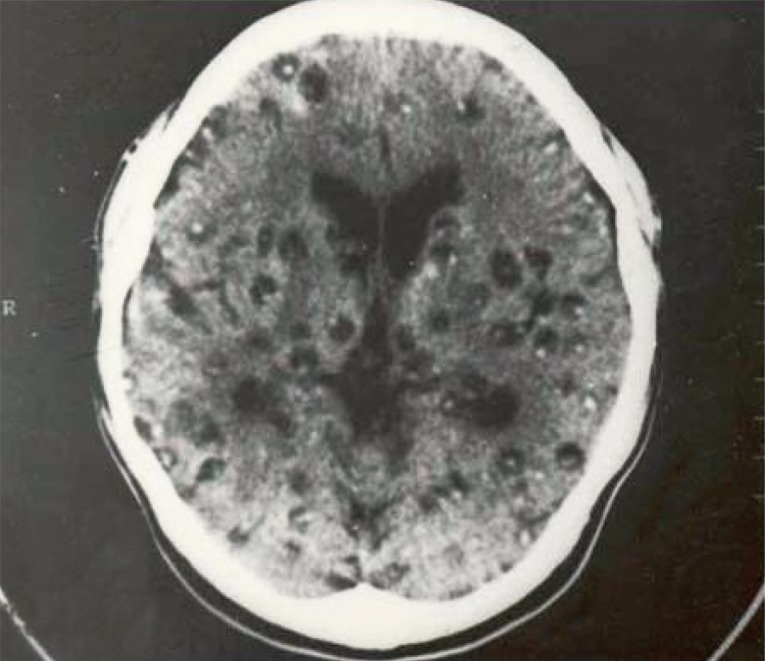
Fig. 5Phylogenetic relationships of the Vietnamese
Taenia and other representative species in the world by analysis of nucleotides using MEGA 2.1 in group A including
Taenia solium and group B including
T. asiatica,
T. saginata, and others including
T. crassiceps (Kumar et al., 2001). TsoCN=
T. solium China; TsoIND=
T. solium India; TsoTL=
T. solium Thailand; TsoINX=
T. solium Indonesia; TsoTZ=
T. solium Tanzania; TsoVN=
T. solium Vietnam; TsoBR=
T. solium Brazil; TsoMX=
T. solium Mexico; TsoEQ=
T. solium Ecuador; TsoCR=
T. solium Cameroon; TasVN1=
T. asiatica Vietnam; TspVN2,3=
T. asiatica Vietnam; TasTW=
T. asiatica Taiwan; TsaCN=
T. saginata China; TspVN4,5,6=
T. saginata Vietnam, and TcrUS=
T. crassiceps America (reproduced from [
8]).