Abstract
There have been some reports on schistosomiasis of school children in Sudan’s Nile River basin area; however, information about the infection status of Schistosoma species and intestinal helminths among village residents of this area is very limited. Urine and stool samples were collected from the 1,138 residents of the Al Hidaib and Khour Ajwal villages of White Nile State, Sudan in 2014. The prevalence of overall schistosomiasis and intestinal helminthiasis was 36.3% and 7.7%, respectively. Egg positive rates were 35.6% for Schistosoma haematobium, 2.6% for S. mansoni, and 1.4% were mixed. The prevalence of schistosomiasis was significantly higher in men (45.6%) than in women (32.0%), in Khou Ajwal villagers (39.4%) than in Al Hidaib villagers (19.2%), and for age groups ≤15 years old (51.5%) than for age groups >15 years old (13.2%). The average number of eggs per 10 ml urine (EP10) of S. haematobium infections was 18.9, with 22.2 eggs in men vs 17.0 in women and 20.4 in Khou Ajwal villagers vs 8.1 in Al Hidaib villagers. In addition to S. mansoni eggs, 4 different species of intestinal helminths were found in the stool, including Hymenolepis nana (6.6%) and H. diminuta (1.0%). Collectively, urinary schistosomiasis is still prevalent among village residents in Sudan’s White Nile River basin and was especially high in men, children ≤15 years, and in the village without a clean water system. H. nana was the most frequently detected intestinal helminths in the 2 villages.
-
Key words: Schistosoma haematobium, Schistosoma mansoni, prevalence, intensity of infection, intestinal helminth, Sudan
INTRODUCTION
Parasitic infections constitute a major health problem worldwide, especially for countries in tropical or subtropical regions. These infections are caused by helminths and protozoa while a variety of conditions contribute to the prevalence of parasitic diseases, such as unsanitary living conditions, inadequate disease control, lack of health education, regional and ethnic customs conducive to parasitic infections, climatic conditions, and compromised immune systems among others [
1,
2]. Schistosomiasis is a water-borne trematode infection. Humans are usually infected by contact with contaminated fresh water, such as during water collection, washing, bathing, playing, fishing, or cultivating crops, and suffer from hematuria and anemia, enlargement of the liver and spleen, and growth retardation [
3]. More than 206.4 million people required preventive chemotherapy for schistosomiasis in 2016, and approximately 200,000 deaths occur due to schistosomiasis globally each year [
4]. Sudan is one of the most highly prevalent countries for schistosomiasis worldwide. There have been several epidemiological surveys of schistosomiasis from Sudanese school aged children in the River Nile State [
5,
6], southern Sudan [
7], Central Sudan [
8], South Kordofan State [
9], and White Nile State [
10–
12]. However, epidemiological surveys of schistosomiasis in village residents are rare.
Intestinal helminthiasis is the most common infections in developing countries including sub-Saharan Africa and East Asia. Intestinal helminthiasis is mainly caused by consumption of contaminated water, infected soil, and inadequate sanitation and hygiene [
1]. Moreover, a lack of access to facilities for safe disposal of human waste can result in intestinal helminthiasis and disease [
13]. High prevalence of intestinal helminthiasis was reported among school children in sub-Saharan African countries [
1,
13,
14]. However, only a few reports were conducted on the prevalence of intestinal helminthiasis in Sudan [
6,
15,
16]; it is also especially difficult to obtain information regarding the infection status of intestinal helminths among village residents in the rural river basin area.
Sudan has wide river basin areas because of the crossings of the Nile Rivers and a large irrigated agriculture sector along the river banks. Due to this geographical environment, schistosomiasis has affected the people of Sudan for centuries. Intestinal helminthiasis may also be prevalent because of the lack of sanitary facilities at rural villages. On account of the lack of reports on schistosomiasis and intestinal helminthiasis in village residents living at the rural river basin area, we conducted this study to investigate the infection status of Schistosoma species and intestinal helminths among village residents of the rural river basin area in Sudan’s White Nile State according to gender, locality, and age groups.
MATERIALS AND METHODS
Study area and population
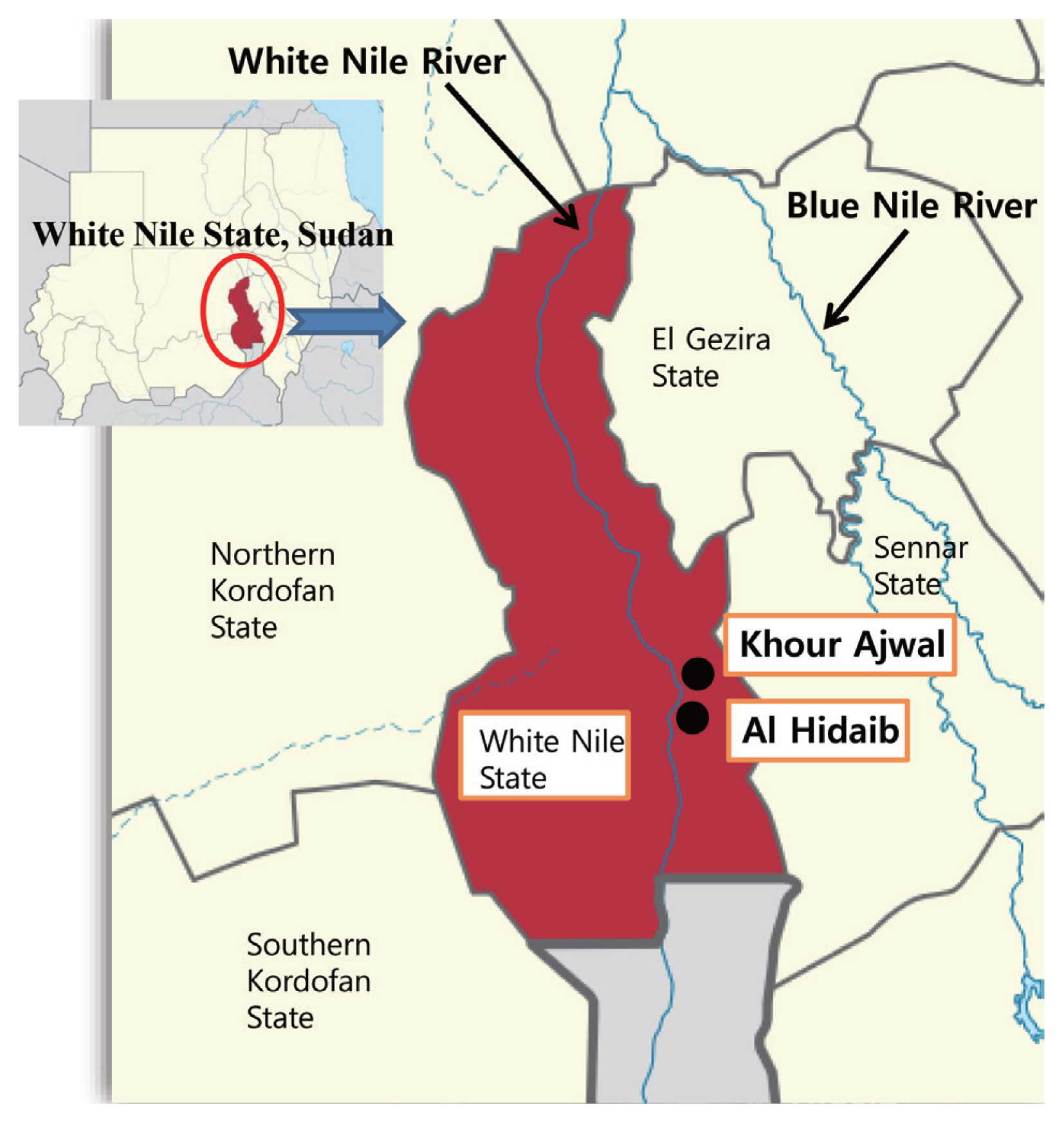
This epidemiological survey was carried out in the Al Hidaib and Khour Ajwal villages in the White Nile State of Sudan, which were located near the White Nile River (
Fig. 1). This survey was conducted by the Korea Association of Health Promotion (KAHP) granted by the Korea International Cooperation Agency (KOICA) from January to February 2014.
A total of 1,138 residents from 2 rural villages were included in this survey (177 individuals from Al Hidaib and 961 individuals from Khou Ajwal;
Table 1). Study participants were 362 men (31.8%) and 776 women (68.2%). Age groups were stratified into the following 4 groups, pre-school-aged children (0–6 years), school-aged children (7–15 years), adolescents and young adults (16–29 years), and older than young adults (≥30 years). Furthermore, age groups were divided into 2 groups of ≤15-year-olds and >15-year-olds. To obtain as many urine and stool samples as possible from both villagers, we divided the 2 villages into 4 sectors (1 sector in Al Hidaib and 3 in Khou Ajwal) considering the distribution of the population and number of health workers in order to increase the efficiency of the work. The researchers who participated in the survey were educated to maintain the consistency of the activities. We also made a list of residents in each sector, and assigned 2 health workers in charge of each sector.
With the help of the village leaders, the purpose of this survey was explained to the village residents and verbal consent was obtained from the village residents, including children and their parents. This study protocol was reviewed and approved by the Institutional Review Board of the KAHP (IRB approval no. 12-C-01). Informed consent was waived by the IRB as the survey was conducted for implementing the integrated schistosomiasis control project by the ODA Project of the Korean government (KOICA project no. 2011-01-010), however we received verbal consents from all village residents participated in this project. At the end of the study, each infected participants were provided with appropriate medicines free of charge for the treatment of schistosomiasis and intestinal parasitic infections.
Collection of urine and stool samples
Each village resident was given 2 coded containers for urine and stool specimens the day before sample collection. The following day after delivering the sample containers, health workers visited each house and collected the samples after permission was obtained through oral consent from each resident. Within 6 hr of urine and stool sample collection from the villages, the samples were transferred to the Schistosomiasis Control Center (established by the KOICA project) in Kosti, a city in the White Nile State.
Parasitological examination
After collection, parasitological examinations were carried out on the same or following day by trained laboratory technicians with more than 5 years of experience in urine and stool analysis. For the detection of
Schistosoma haematobium eggs, the urine sedimentation method was employed. Briefly, the amount of urine in each tube was adjusted to 10 ml and then centrifuged at 1,500 rpm for 5 min at room temperature. The supernatant fluid was decanted and then sediments were transferred onto 2–4 glass slides to examine the whole tube pellet as much as possible; all slides were imaged via microscopy to detect
S. haematobium eggs. Infection intensities of
S. haematobium were classified into 2 categories, light (eggs per 10 ml urine [EP10]<50) and heavy (EP10≥50) infections [
8].
For the detection of
S. mansoni and other intestinal helminths, stool samples were subjected to the Kato cellophane thick smear method [
17]. Briefly, approximately 100 mg of fecal sample was obtained from each tube and then 2 smears were prepared on glass slides and allowed to clear for at least 30 min using cellophane that soaked in 0.018% malachite green and 3% phenol prior to examination. The presence of
S. mansoni eggs or intestinal parasites for each person was the sum of 2 smears.
For quality control, 10% of slides were randomly selected and re-examined at the end of each day by parasitology experts that was blinded to the results of the first examination. In case of disagreement, the results were discussed with the concerned technician and the discordant slides were re-examined until an agreement was reached.
Statistical analysis
SPSS version 16.0 software (SPSS Inc., San Diego, California, USA) was used to analyze the experimental data. Due to the deviation from normality distribution, infection intensity (EP10) was expressed as geometric mean and median. The differences in continuous variables among groups were tested using a 2-tailed Mann-Whitney U test, Kruskal-Wallis test, and Student’s t-test. The association between study variables and parasitic infections were tested using logistic regression analysis. Odds ratio (OR) and 95% confidence interval (CI) were also calculated and P-values<0.05 were considered statistically significant.
RESULTS
Sample collection status and overall prevalence of schistosomiasis
As shown in
Table 2, a total of 1,131 urine and 1,038 stool samples were collected from 1,138 village residents. Among all sample donors, 1,031 individuals presented both urine and stool samples, while only 107 individuals presented only one sample of either urine or stool.
As a result of parasitological examination, 413 of 1,138 individuals were found infected with
S. haematobium,
S. mansoni, or both and thus the overall prevalence of infections with
Schistosoma species was 36.3%. Prevalence of
S. haematobium and
S. mansoni infections were 35.6% and 2.6%, respectively (
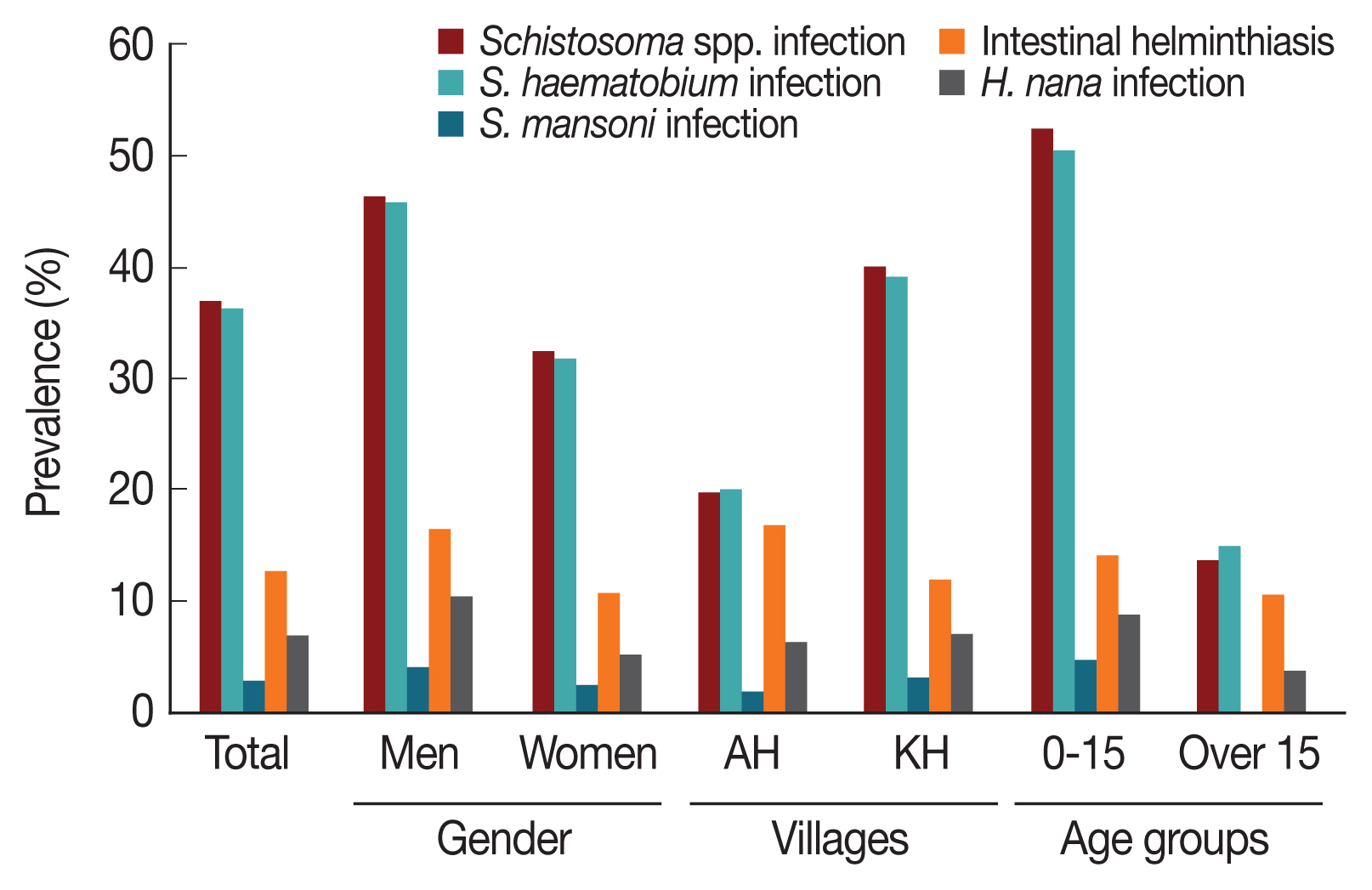
Fig. 2), while 1.4% were mixed.
Table 3 shows the association between risk factors and schistosomiasis in univariate analyses. The prevalence of schistosomiasis was significantly different (
P<0.0001; 95% CI=1.372–2.290) between men and women a 45.6% and 32.0%, respectively. Schistosomiasis prevalence was also significantly different (
P<0.0001; 95% CI=1.852–4.086) between the 2 villages, presenting with 19.2% in Al Hidaib villagers and 34.9% in Khour Ajwal villagers. According to the stratified age groups, the highest prevalence was 53.2% in school-aged children (7–15 years), followed by 48.2% in pre-school-aged children (0–6 years). The prevalence was also significantly higher in the ≤15-year-old (51.5%) group than in the >15-year-old (13.2%) group (
P<0.0001; 95% CI=5.046–9.355). In multivariate analysis, factors that remained significantly associated with schistosomiasis were locality (
P<0.0001; OR=3.446; 95% CI=2.272–5.226) and age group (
P<0.0001; OR=7.122; 95% CI=5.159–9.831), but not gender (
P<0.3; OR=1.164; 95% CI=0.873–1.552;
Table 4).
As shown in
Fig. 2, the prevalence of
S. haematobium infections was 35.6% (403 out of 1,131 cases). The
S. haematobium infection rate of men (45.0%) was significantly higher than that of women (31.2%;
P<0.0001; 95% CI=1.391–2.325;
Table 3). Based on locality, the prevalence of
S. haematobium infections in Al Hidaib villagers (19.4%) was significantly lower than that of Khour Ajwal villagers (38.5%;
P<0.0001; 95% CI=1.831–4.075). The prevalence by age group was 51.1% for 0–6-year-olds, 48.9% for 7–15-year-olds, 20.0% for 16–29-year-olds, and 10.7% for those older than 30 years. Significant differences were also observed for the prevalence between the ≤15-year-old (49.6%) and >15-year-old (14.4%) groups (
P<0.0001; 95% CI=4.843–9.008). In multivariate analysis, factors that remained significantly associated with
S. haematobium infection were locality (
P<0.0001; OR=3.384; 95% CI=2.224–5.149) and age group (
P<0.0001; OR=6.767; 95% CI=4.897–9.351), but not gender (
P<0.217; OR=1.198; 95% CI=0.899–1.597;
Table 4).
To check the intensity of
S. haematobium-infected individuals, we counted the number of
S. haematobium EP10 and expressed the intensity as geometric mean (
Table 5). The average geometric mean of
S. haematobium-infected individuals was 18.9 EP10 (range, 2–800 EP10). The geometric mean for men was 22.2 EP10 (range, 2–800 EP10) and 17 EP10 for women (range, 2–600 EP10), exhibiting significant differences (
P<0.0001;
Table 5). The geometric mean for Khou Ajwal villagers (20.4 EP10; range, 2–800 EP10) was significantly higher than that of the Al Hidaib villagers (8.1 EP10; range, 2–60 EP10;
P<0.0001;
Table 5). Furthermore, based on age groups, the highest geometric mean was observed for school-aged children (20.5 EP10; range, 2–800 EP10), followed by pre-school-aged children (19.6 EP10; range, 2–600 EP10). Significant differences in geometric mean intensity between the ≤15-year-old (20.2 EP10) and >15-year-old (12.9 EP10) age groups were observed (
P<0.0001;
Table 5). Of all
S. haematobium-infected individuals, 71.0% presented with light infections (EP10<50) while 29.0% were considered heavily infected (EP10≥50).
S. mansoni eggs in stool were found for 27 individuals among 1,013 residents that were between 0–29 years old; thus, the prevalence of
S. mansoni infections was 2.6% (
Fig. 2). Prevalence according to gender was 3.6% in men and 2.1% in women, which was not significantly different (
P=0.159;
Fig. 2). A higher prevalence was observed for Khou Ajwal villagers (2.8%) than for Al Hidaib villagers (1.5%), but there was no significant difference (
P=0.251;
Table 3). Most
S. mansoni-infected individuals were ≤15 years old (4.1%), exhibiting significantly higher prevalence than that of individuals >15 years old (0.2%;
P=0.005; 95% CI=2.420–132.378;
Table 3).
Prevalence of intestinal helminthiasis
Besides
S. mansoni, 4 different intestinal helminths were found among the 1,038 stool samples including
Hymenolepis nana (68 cases),
H. diminuta (8 cases),
S. haematobium (2 cases), and
Taenia saginata (2 cases). Thus, overall prevalence of intestinal helminthiasis in stool (besides
S. mansoni eggs) were 7.7% (80/1,038 cases;
Fig. 2). As shown in
Table 6, the prevalence in men and women was significantly different (
P=0.003; 95% CI=1.236–3.267) at 11.7% and 5.8%, respectively. Prevalence in Al Hidaib villagers (6.7%) was lower than in Khou Ajwal villagers (7.9%;
P=0.393). By age group, prevalence was the highest for pre-school-aged children (11.2%), followed by school-aged children (9.3%) and adults older than 30 years (5.2%); there were no significant differences of prevalence of intestinal helminthiasis among age groups. There were significant differences of the prevalence between the ≤15-year-old (10.0%) and ≤15-year-old (4.2%) groups (
P<0.001; 95% CI=1.438–4.659). Among intestinal helminths found in stool besides
S. mansoni (2.6%),
H. nana (6.6%) was the most prevalent, followed by
H. diminuta (1.0%),
S. haematobium (0.2%), and
T. saginata (0.2%). As shown in
Table 6 and
Fig. 2, the prevalence of
H. nana infections was significantly higher in men and individuals >15 years old than for women and individuals >15 years old (gender,
P=0.003, 95% CI=1.297–3.477; age group,
P=0.001, 95% CI=1.478–4.910). The highest prevalence of
H. nana infections was observed in pre-school-aged children (9.8%), followed by school-aged children (7.9%) and adults older than 30 years (4.4%). There were no significant differences of
H. nana infection prevalence between the Al Hidaib (5.9%) and Khou Ajwal (6.6%) villages (
P=0.376). In multivariate analysis, factors that remained significantly associated with
H. nana infection were gender (
P=0.008; OR= 1.733; 95% CI=1.041–2.884) and age group (
P=0.034; OR= 2.314; 95% CI=1.246–4.299), but not locality (
Table 4).
DISCUSSION
Schistosomiasis is the second most common neglected tropical disease (NTD) after soil-transmitted helminths (STH) infections in sub-Saharan Africa. Sudan is also located in sub-Saharan Africa and human schistosomiasis is of considerable public health importance in the country. In school children-based surveys of schistosomiasis in Sudan, the prevalence of
S. haematobium and
S. mansoni infections were 73% and 70%, respectively, in the Upper Nile region [
7] and 23.7% and 0%, respectively, in South Kordofan State [
9]. In White Nile State, egg positive rates of
S. haematobium and
S. mansoni were 45.0% and 5.9%, respectively [
11], and were 28.5% and 0.4%, respectively [
12]. Community-based studies in Sudan reported that the prevalence of
S. mansoni infections was 27.4% in villagers of the New Halfa Agricultural Scheme of Kassala State, but no data on
S. haematobium was provided [
18]. These results indicate that there is a wide variation in the distribution of
Schistosoma species and schistosomiasis prevalence depending on survey sites. The variations may be explained by variable ecological conditions of surveyed sites, socioeconomic conditions of the surveyed populations, control programs implemented, the study design itself, and so on. In the present study, the prevalence of
S. haematobium and
S. mansoni infections was 35.6% and 2.6%, respectively, in the White Nile River basin area. Throughout the Nile basin of Sudan,
S. haematobium is predominantly transmitted compared with
S. mansoni [
9–
12]. Across Sudan however,
S. mansoni infections were more prevalent in the Kassala State only [
18]; this difference may be due to the snail ecology of Kassala State. Furthermore, compared with the reported prevalence in the White Nile State, our study provided
S. haematobium infection rates of 49.6% in the ≤15 age group, which was higher than the previous reports of 21.4% [
10], 45.0% [
11], and 28.5% for school children [
12]. Moreover, our reported prevalence of
S. haematobium infection among village residents (35.6%) was higher than the 14.0% previously reported [
19] for villagers in the White Nile State. These findings suggest that village residents—both children and adults—in these 2 rural river basin areas have been continuously in contact with
S. cercariae-contaminated water despite the control measures that have been implemented in the White Nile State.
The current study demonstrated that the prevalence of schistosomiasis was higher in men and individuals ≤15 years old than in women and individuals >15 years old. This finding agrees with several previous studies conducted in endemic areas [
5,
12,
18,
20]. The association between gender and schistosomiasis may be attributed to religious and sociocultural reasons or to water contact behavior. In Sudan and many other Muslim countries, women are prohibited from bathing in open water sources, whereas the men frequently play and swim during their leisure time and thus schistosomiasis prevalence among men is significantly higher [
5,
12,
18,
21,
22]. However, some studies have reported no significant differences between the genders because women are also responsible for fetching water and washing clothes and utensils at these water sources and thus have similar exposure to men [
10,
11,
23]. Meanwhile, according to the stratified age groups, higher schistosomiasis prevalence was observed for those ≤15 years old, which includes pre-school and school-aged children. This finding is consistent with previous reports from different endemic areas [
5,
10,
20]. In most endemic areas, schistosomiasis prevalence increases with age up to 10–15 years old, followed by a decline in older ages [
5,
20]. This could be explained by the fact that children of this age are more mobile and often go into the water near the village either to assist their parents in agricultural activities or to swim and play in the canals, which are likely to be contaminated with infective stages of schistosomiasis or other parasitic diseases [
5,
11].
In this study, we also compared the infection status of
Schistosoma species between the Al Hidaib and Khour Ajwal villages, which are neighboring rural communities adjacent to the White Nile River and have very similar ecological conditions. However, our reported prevalence of schistosomiasis in Khour Ajwal was significantly higher than in Al Hidaib. This may be due to several factors; one critical difference is that a large, clean water supply system was installed in the Al Hidaib village and has been in use for many years, whereas there is no clean water supply system for Khour Ajwal and thus residents used river water. Consistent with our results, a previous study also reported that a facility for clean drinking water in Al Hidaib contributed to the improvement in health and life quality of the village residents by preventing waterborne diseases, including schistosomiasis [
12]. Furthermore, the use of water purification systems was critical in reducing
Cryptosporidium infections, a type of water borne pathogen, among inhabitants [
16].
Infection intensity is a better indicator of morbidity than schistosomiasis prevalence because disease progression is associated with the daily deposition of parasite eggs into host tissues [
8,
24]. In this study, we used urine sedimentation method to detect and quantify the
S. haematobium eggs. That is why urine filtration system is blocked easily due to many foreign substances in the urine collected at the survey sites (such as fallen bladder cells and so on, and the associated paper is Reference [
19]), and it is higher detectability even if low number of parasites, and it is also economic than urine filtration method. According to previous reports from Sudan, the intensities as mean EP10 of
S. haematobium were 12.9 in White Nile State [
10], 25.5 in South Darfur [
25], 87.7 in Central Sudan [
8], 55 in White Nile State [
11] and 40.1 in River Nile State [
5]. In the present study, the intensity average geometric mean, median, and mean±standard deviation (SD) were 18.9, 20, and 67.78±119.49 EP10 (range, 2–800 EP10), respectively, in
S. haematobium-infected individuals and 71% were light infections (EP10<50). Compared with the previously reported intensities of urinary schistosomiasis in Sudan, our results were similar or little less with those for the White Nile State [
10,
11]—albeit exact comparisons are difficult to achieve. We also analyzed
S. haematobium infection intensities according to gender, locality, and age group and found that the tendency of
S. haematobium infection intensities was in line with prevalence by gender, locality, and age group. The intensity geometric mean of men (22.2 EP10) was significantly higher than that of women (17.0 EP10), which was consistent with other reports [
5,
26]. On the other hand, Afifi et al. [
18] reported that the intensity of
S. mansoni infections among women (293.4 EPG) was higher than in men (187.6 EPG), although prevalence of infection among men (41.4%) was much higher than for women (13.9%). Moreover, based on age groups, we found that the intensity geometric mean of individuals ≤15 years old (20.2 EP10) was significantly higher than for individuals >15 years old (12.9 EP10). However, it was reported that high
S. mansoni infection intensity was present in the age groups 31–40 and >50 years [
18].
Intestinal parasitic infections constitute a major health burden in many developing countries. People of all ages can be affected by intestinal parasitic infections, with an increased risk for school children [
1,
27]. Apart from causing morbidity and mortality, infections with intestinal parasites have considerable impact on normal development, well-being, and the cognitive and educational performance of school-aged children [
1]. According to previous studies conducted in Sudan, the prevalence of intestinal helminths was 2.7% in Khartoum State [
28], while infection rates of
H. nana were 32.6% in Khartoum State [
15]. In the present study, the overall prevalence of intestinal helminthiasis was 7.7%, which was higher than the report of pre-school-aged children in Khartoum State [
15]. And we found 5 different species of intestinal helminths including
S. mansoni, which was the similar number of helminth’s species reported by Babiker et al. [
28]. We found that the highest prevalence among intestinal parasitic infections was
H. nana infections (6.6%).
H. nana commonly infects both humans and rodents and can have an epidemiologically significant impact in family units, because it is the only tapeworm that can be directly transmitted between humans and auto-reinfection is possible [
15,
29]. Our reported
H. nana infection rate was higher than for food handlers (1.6%) [
28] and much lower among pre-school-aged children (32.6%) [
15] in Sudan. These variations may be due to differences in climatic conditions, water sources, health and environmental sanitation, previous control interventions, socioeconomic status of the population subjects, and differences in host susceptibility to parasitic infections [
15,
29]. Abdel Hamnid et al. [
15] reported that
H. nana infections were more prevalent among men than women, with no significant differences between age groups, which was also observed in this study. Interestingly, there were no significant differences in the prevalence of intestinal helminthiasis between Al Hidaib and Khour Ajwal villages, even though schistosomiasis prevalence was significantly higher in Al Hidaib than in Khou Ajwal. Thus, major intestinal helminths detected in this study,
H. nana, are more affected by the general environmental sanitation than access to clean water supplies.
The limitation of this study is based on the single egg screening process, which is less reliable in estimating prevalence of schistosomiasis and intestinal parasitic infections. The examination of 2 or more specimens at different time points for every individual would likely have resulted in higher prevalence for schistosomiasis and intestinal helminthiasis as well as a larger number of detected intestinal parasite species. In addition, we applied Kato Cellophane method to examine the stool samples, thus there were some limitation to detect the intestinal protozoa.
Our data provide up-to-date information regarding the infection status of Schistosoma species and intestinal helminths among village residents living in the rural river basin area in Sudan’s White Nile State. From this study, the overall prevalence of schistosomiasis and intestinal helminthiasis was found to be 36.3% and 7.7%, respectively. Urinary schistosomiasis was found highly prevalent among pre-school and school-aged children, and intestinal helminths are also prevalent among the village residents. Therefore, integrated intervention including health education, environmental hygiene, and clean water supplies and treatment should be taken into account to reduce the prevalence of schistosomiasis and intestinal parasitic infections.
Notes
-
CONFLICT OF INTERESTS
The authors declare that they have no competing interests.
ACKNOWLEDGMENTS
We appreciate all the staffs involved in “The Project for Combating Schistosomiasis in Sudan,” carried by Korea Association of Health Promotion (KAHP) and the Ministry of Health (MoH) of the White Nile State, Sudan. We are indebted to several colleagues at Ministry of Health of Sudan and Embassy of the Republic of Korea in Sudan. Also we thank all village residents, including children and their parents, of Al Hidaib and Khour Ajwal villages of White Nile State, Sudan. This project was supported by the Korea International Cooperation Agency (KOICA project no. P2015-00146-2).
Fig. 1Location of the Al Hidaib and Khour Ajwal villages of White Nile State, Sudan.

Fig. 2Comparison of prevalence of Schistosomiasis and intestinal helminthiasis among rural village residents of the White Nile River basin area in Sudan according to gender, locality, and age group.
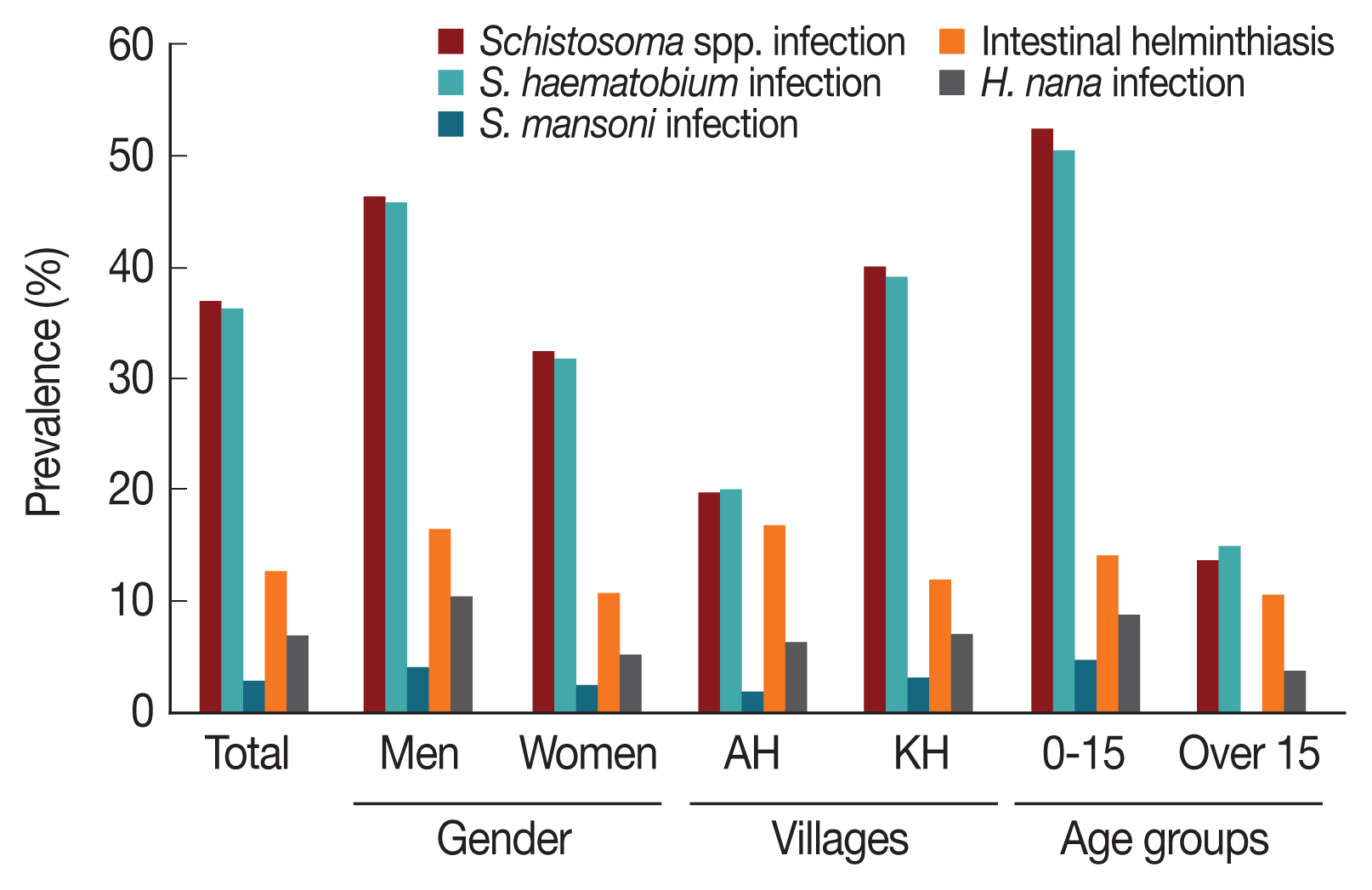
Table 1Locality, gender, and age group distribution of the surveyed population in the White Nile River basin area in Sudan
Table 1
|
Village |
Gender |
Age in year (%) |
|
|
≥ 6 |
7–15 |
16–29 |
≥30 |
Total |
|
Al Hidaib |
Men |
11 |
34 |
10 |
14 |
69 |
|
Women |
8 |
60 |
22 |
18 |
108 |
|
Subtotal |
19 |
94 |
32 |
32 |
177 (15.6) |
|
|
Khou Ajwal |
Men |
106 |
137 |
19 |
31 |
293 |
|
Women |
93 |
235 |
131 |
209 |
668 |
|
Subtotal |
199 |
372 |
150 |
240 |
961 (84.5) |
|
|
Total |
Men |
117 |
171 |
29 |
45 |
362 (31.8) |
|
Women |
101 |
295 |
153 |
227 |
776 (68.2) |
|
Total |
218 (19.2) |
466 (41.0) |
182 (16.0) |
272 (23.9) |
1,138 (100.0) |
Table 2Sample numbers lists collected from rural village residents of the White Nile River basin area in Sudan for the examination of schistosomiasis and intestinal parasitic infections
Table 2
|
Village |
Gender |
No. of samples (%) |
|
|
Urine |
Stool |
Urine and stool |
Total |
|
Al Hidaib |
Men |
67 |
52 |
50 |
69 |
|
Women |
103 |
83 |
78 |
108 |
|
Subtotal |
170 |
135 |
128 |
177 (15.6) |
|
|
Khou Ajwal |
Men |
293 |
281 |
281 |
293 |
|
Women |
668 |
622 |
622 |
668 |
|
Subtotal |
961 |
903 |
903 |
961 (84.5) |
|
|
Total |
Men |
360 |
333 |
331 |
362 (31.8) |
|
Women |
771 |
705 |
700 |
776 (68.2) |
|
Total |
1,131 (99.4) |
1,038 (91.2) |
1,031 (90.6) |
1,138 (100.0) |
Table 3Univariate analysis of factors associated with Schistosoma infections among rural village residents of the White Nile River basin area in Sudan
Table 3
|
Variables |
No. exam. |
Schistosoma species infection |
S. haematobium infection |
S. mansoni infection |
|
|
|
|
No. of positive (%) |
OR (95% CI) |
P-value |
No. of positive (%) |
OR (95% CI) |
P-value |
No. of positive (%) |
OR (95% CI) |
P-value |
|
Gender |
|
Men |
362 |
165 (45.6) |
1.773 (1.372–2.290) |
<0.0001 |
162 (45.0) |
1.798 (1.391–2.325) |
<0.0001 |
12 (3.6) |
1.739 (0.806–3.755) |
0.159 |
|
Women |
776 |
248 (32.0) |
1 |
|
241 (31.2) |
1 |
|
15 (2.1) |
1 |
|
|
|
Villages |
|
Al Hidaib |
177 |
34 (19.2) |
1 |
|
33 (19.4) |
1 |
|
2 (1.5) |
1 |
|
|
Khou Ajwal |
961 |
379 (39.4) |
2.751 (1.852–4.086) |
<0.0001 |
370 (38.5) |
2.732 (1.831–4.075) |
<0.0001 |
25 (2.8) |
2.337 (0.549–9.956) |
0.251 |
|
|
Age in year (I) |
|
≤6 |
218 |
105 (48.2) |
8.714 (5.432–13.980) |
<0.0001 |
111 (51.1) |
8.097 (5.047–12.992) |
<0.0001 |
8 (3.9) |
- |
0.994 |
|
7–15 |
466 |
248 (53.2) |
9.578 (6.223–14.742) |
<0.0001 |
227 (48.9) |
9.096 (5.910–14.000) |
<0.0001 |
18 (4.2) |
- |
0.994 |
|
16–29 |
182 |
32 (17.6) |
1.930 (1.121–3.323) |
0.018 |
36 (20.0) |
1.859 (1.076–3.211) |
0.026 |
1 (0.6) |
- |
0.995 |
|
≥30 |
272 |
28 (10.3) |
1 |
|
29 (10.7) |
1 |
|
0 (0.0) |
1 |
|
|
|
Age in year (II) |
|
≤15 |
684 |
353 (51.5) |
6.871 (5.046–9.355) |
<0.0001 |
338 (49.6) |
6.605 (4.843–9.008) |
<0.0001 |
26 (4.1) |
17.900 (2.420–132.378) |
0.005 |
|
>15 |
454 |
60 (13.2) |
1 |
|
65 (14.4) |
1 |
|
0 (0.0) |
1 |
|
Table 4Multivariate analysis of factors associated with Schistosoma and H. nana infections among rural village residents of the White Nile River basin area in Sudan
Table 4
|
Variables |
Schistosoma species infection |
S. haematobium infection |
H. nana infection |
|
|
|
|
Adjusted OR |
95% CI |
P-value |
Adjusted OR |
95% CI |
P-value |
Adjusted OR |
95% CI |
P-value |
|
Gender |
1.164 |
0.873–1.552 |
0.300 |
1.198 |
0.899–1.597 |
0.217 |
1.733 |
1.041–2.884 |
0.008 |
|
|
Villages |
3.446 |
2.272–5.226 |
<0.001 |
3.384 |
2.224–5.149 |
<0.001 |
- |
- |
- |
|
|
Age in year (II) |
7.122 |
5.159–9.831 |
<0.001 |
6.767 |
4.897–9.351 |
<0.001 |
2.314 |
1.246–4.299 |
0.034 |
Table 5Prevalence and intensity of S. haematobium infection among rural village residents of the White Nile River basin area in Sudan according to gender, locality, and age group
Table 5
|
Variable |
No. exam. |
No. of positive (%) |
Geometric mean |
P-value |
|
Gender |
|
Men |
360 |
162 (45.0) |
22.2 |
<0.001 |
|
Women |
771 |
241 (31.2) |
17.0 |
|
|
|
Villages |
|
Al Hidaib |
170 |
33 (19.4) |
8.1 |
<0.001 |
|
Khou Ajwal |
961 |
370 (38.5) |
20.4 |
|
|
|
Age in year (I) |
|
≤6 |
217 |
111 (51.1) |
19.6 |
<0.001 |
|
7–15 |
464 |
227 (48.9) |
20.5 |
|
|
16–29 |
180 |
36 (20.0) |
11.9 |
|
|
≥30 |
270 |
29 (10.7) |
14.1 |
|
|
|
Age in year (II) |
|
≤15 |
681 |
338 (49.6) |
20.2 |
<0.001 |
|
>15 |
450 |
65 (14.4) |
12.9 |
|
Table 6Univariate analysis of factors associated with intestinal parasitic infections among rural village residents of the White Nile River basin area in Sudan
Table 6
|
Variable |
No. exam. |
Intestinal helminthiasis*
|
H. nana infection |
|
|
|
No. of positive (%) |
OR (95% CI) |
P-value |
No. of positive (%) |
OR (95% CI) |
P-value |
|
Gender |
|
Men |
333 |
39 (11.7) |
2.0143 (1.236–3.267) |
0.003 |
33 (10.0) |
2.124 (1.297–3.477) |
0.003 |
|
Women |
705 |
41 (5.8) |
1 |
|
35 (5.0) |
1 |
|
|
|
Villages |
|
Al Hidaib |
135 |
9 (6.7) |
1 |
|
8 (5.9) |
1 |
|
|
Khou Ajwal |
903 |
71 (7.9) |
0.896 (0.529–1.411) |
0.393 |
60 (6.6) |
1.407 (0.661–2.995) |
0.376 |
|
|
Age in year (I) |
|
≤6 |
205 |
23 (11.2) |
2.238 (1.218–4.832) |
0.027 |
20 (9.8) |
2.397 (1.122–5.117) |
0.024 |
|
7–15 |
428 |
40 (9.3) |
1.843 (0.965–3.849) |
0.083 |
34 (7.9) |
1.867 (0.930–3.749) |
0.079 |
|
16–29 |
154 |
4 (2.6) |
0.369 (0.127–1.538) |
0.123 |
3 (1.9) |
0.398 (0.109–1.446) |
0.161 |
|
≥30 |
251 |
13 (5.2) |
1 |
|
11 (4.4) |
1 |
|
|
|
Age in year (II) |
|
≤15 |
633 |
63 (10.0) |
2.586 (1.438–4.659) |
0.001 |
54 (8.5) |
2.694 (1.478–4.910) |
0.001 |
|
>15 |
405 |
17 (4.2) |
1 |
|
14 (3.5) |
1 |
|
References
- 1. Alum A, Rubino JR, Ijaz MK. The global war against intestinal parasites-should we use a holistic approach? Int J Infect Dis 2010;14:e732-738.
- 2. Lo NC, Addiss DG, Hotez PJ, King CH, Stothard JR, Evans DS, Colley DG, Lin W, Coulibaly JT, Bustinduy AL, Raso G, Bendavid E, Bogoch II, Fenwick A, Savioli L, Molyneux D, Utzinger J, Andrews JR. A call to strengthen the global strategy against schistosomiasis and soil-transmitted helminthiasis: the time is now. Lancet Infect Dis 2017;17:64-69.
- 3. Adenowo AF, Oyinloye BE, Ogunyinka BI, Kappo AP. Impact of human schistosomiasis in sub-Saharan Africa. Braz J Infect Dis 2015;19:196-205.
- 4. World Health Organization. Fact Sheet on Schistosomiasis. [Internet]; Available from: http://www.who.int/mediacentre/factsheets/fs115/en/
- 5. Sulieman Y, Eltayeb RE, Pengsakul T, Afifi A, Zakaria MA. Epidemiology of urinary schistosomiasis among school children in the Alsaial Alsagair village, River Nile State, Sudan. Iran J Parasitol 2017;12:284-291.
- 6. Elmadhoun WM, Msmar AH, Elnoby OA, Noor SK, Suliman AA, Bushara SO. Situation analysis of schistosomiasis and soil-transmitted helminthes in River Nile State, Sudan. Trans R Soc Trop Med Hyg 2013;107:195-199.
- 7. Deganello R, Cruciani M, Beltramello C, Duncan O, Oyugi V, Montresor A.
Schistosoma hematobium and S. mansoni among children, Southern Sudan. Emerg Infect Dis 2007;13:1504-1506.
- 8. Ahmed AM, Abbas H, Mansour FA, Gasim GI, Adam I.
Schistosoma haematobium infections among schoolchildren in central Sudan one year after treatment with praziquantel. Parasit Vectors 2012;5:108.
- 9. Abou-Zeid AH, Abkar TA, Mohamed RO. Schistosomiasis infection among primary school students in a war zone, Southern Kordofan State, Sudan: a cross-sectional study. BMC Public Health 2013;13:643.
- 10. Ahmed ES, Daffalla A, Christensen NO, Madsen H. Patterns of infection and transmission of human schistosomiasis mansoni and schistosomiasis haematobium in White Nile Province, Sudan. Ann Trop Med Parasitol 1996;90:173-180.
- 11. Ismail HA, Hong ST, Babiker AT, Hassan RM, Sulaiman MA, Jeong HG, Kong WH, Lee SH, Cho HI, Nam HS, Oh CH, Lee YH. Prevalence, risk factors, and clinical manifestations of schistosomiasis among school children in the White Nile River basin, Sudan. Parasit Vectors 2014;7:478.
- 12. Lee YH, Jeong HG, Kong WH, Lee SH, Cho HI, Nam HS, Ismail HA, Alla GN, Oh CH, Hong ST. Reduction of urogenital schistosomiasis with an integrated control project in Sudan. PLoS Negl Trop Dis 2015;9:e3423.
- 13. Erismann S, Diagbouga S, Odermatt P, Knoblauch AM, Gerold J, Shrestha A, Grissoum T, Kaboré A, Schindler C, Utzinger J, Cissé G. Prevalence of intestinal parasitic infections and associated risk factors among schoolchildren in the Plateau Central and Centre-Ouest regions of Burkina Faso. Parasit Vectors 2016;9:554.
- 14. Hailegebriel T. Prevalence of intestinal parasitic infections and associated risk factors among students at Dona Berber primary school, Bahir Dar, Ethiopia. BMC Infect Dis 2017;17:362.
- 15. Abdel Hamid MM, Eljack IA, Osman MK, Elaagip AH, Muneer MS. The prevalence of Hymenolepis nana among preschool children of displacement communities in Khartoum state, Sudan: a cross-sectional study. Travel Med Infect Dis 2015;13:172-177.
- 16. Sim S, Yu JR, Lee YH, Lee JS, Jeong HG, Mohamed AA, Hong ST. Prevalence of Cryptosporidium infection among inhabitants of 2 rural areas in White Nile State, Sudan. Korean J Parasitol 2015;53:745-747.
- 17. Hong SJ, Woo HC, Han JH, Kim HJ. Comparative study on the effectiveness of modified Kato’s cellophane thick smear and Stoll’s dilution egg counting technique for quantitative fecal examination of helminth eggs. Korean J Parasitol 1992;30:141-145. (in Korean).
- 18. Afifi A, Ahmed AA, Sulieman Y, Pengsakul T. Epidemiology of schistosomiasis among villagers of the New Halfa Agricultural Scheme, Sudan. Iran J Parasitol 2016;11:110-115.
- 19. Kim MJ, Ryu K, Jin Y, Lee YH, Jeoung HG, Saeed AA, Kim SH, Hong ST. Significance of echogenic snow sign as an ultrasonography finding for diagnosis of urogenital schistosomiasis. Am J Trop Med Hyg 2016;95:842-848.
- 20. Deribe K, Eldaw A, Hadziabduli S, Kailie E, Omer MD, Mohammed AE, Jamshed T, Mohammed EA, Mergani A, Ali GA, Babikir K, Adem A, Hashim F. High prevalence of urinary schistosomiasis in two communities in South Darfur: implication for interventions. Parasit Vectors 2011;4:14.
- 21. Kapito-Tembo AP, Mwapasa V, Meshnick SR, Samanyika Y, Banda D, Bowie C, Radke S. Prevalence distribution and risk factors for Schistosoma hematobium infection among school children in Blantyre, Malawi. PLoS Negl Trop Dis 2009;3:e361.
- 22. Atalabi TE, Adoh SD, Eze KM. The current epidemiological status of urogenital schistosomiasis among primary school pupils in Katsina State, Nigeria: An imperative for a scale up of water and sanitation initiative and mass administration of medicines with Praziquantel. PLoS Negl Trop Dis 2018;12:e0006636.
- 23. Sady H, Al-Mekhlafi HM, Mahdy MA, Lim YA, Mahmud R, Surin J. Prevalence and associated factors of schistosomiasis among children in Yemen: implications for an effective control programme. PLoS Negl Trop Dis 2013;7:e2377.
- 24. Andrade G, Bertsch DJ, Gazzinelli A, King CH. Decline in infection-related morbidities following drug-mediated reductions in the intensity of Schistosoma infection: A systematic review and meta-analysis. PLoS Negl Trop Dis 2017;11:e0005372.
- 25. Ahmed AA, Afifi AA, Adam I. High prevalence of Schistosoma haematobium infection in Gereida Camp, in southern Darfur, Sudan. Ann Trop Med Parasitol 2009;103:741-743.
- 26. Senghor B, Diallo A, Sylla SN, Doucouré S, Ndiath MO, Gaayeb L, Djuikwo-Teukeng FF, Bâ CT, Sokhna C. Prevalence and intensity of urinary schistosomiasis among school children in the district of Niakhar, region of Fatick, Senegal. Parasit Vectors 2014;7:5.
- 27. Forson AO, Arthur I, Ayeh-Kumi PF. The role of family size, employment and education of parents in the prevalence of intestinal parasitic infections in school children in Accra. PLoS One 2018;13:e0192303.
- 28. Babiker MA, Ali MS, Ahmed ES. Frequency of intestinal parasites among food-handlers in Khartoum, Sudan. East Mediterr Health J 2009;15:1098-1104.
- 29. Vilchez Barreto PM, Gamboa R, Santivañez S, O’Neal SE, Muro C, Lescano AG, Moyano LM, Gonzálvez G, García HH; The Cysticercosis Working Group In Perú. Prevalence, age profile, and associated risk factors for Hymenolepis nana infection in a large population-based study in Northern Peru. Am J Trop Med Hyg 2017;97:583-586.