Abstract
We conducted a study to compare the safety and tolerability of anti-relapse drugs elubaquine and primaquine against Plasmodium vivax malaria. After standard therapy with chloroquine, 30 mg/kg given over 3 days, 141 patients with P. vivax infection were randomized to receive primaquine or elubaquine. The 2 treatment regimens were primaquine 30 mg once daily for 7 days (group A, n = 71), and elubaquine 25 mg once daily for 7 days (group B, n = 70). All patients cleared parasitemia within 7 days after chloroquine treatment. Among patients treated with primaquine, one patient relapsed on day 26; no relapse occurred with elubaquine treatement. Both drugs were well tolerated. Adverse effects occurred only in patients with G6PD deficiency who were treated with primaquine (group A, n = 4), whose mean hematocrit fell significantly on days 7, 8 and 9 (P = 0.015, 0.027, and 0.048, respectively). No significant change in hematocrit was observed in patients with G6PD deficiency who were treated with elubaquine (group B, n = 3) or in patients with normal G6PD. In conclusion, elubaquine, as anti-relapse therapy for P. vivax malaria, was as safe and well tolerated as primaquine and did not cause clinically significant hemolysis.
-
Key words: Plasmodium vivax, vivax malaria, elubaquine, bulaquine, safety, tolerability, Thailand
INTRODUCTION
Plasmodium vivax causes an acute, debilitating febrile illness in which the primary infection may be followed by relapses originating from hypnozoites, dormant liver stages of the parasite (
Baird and Hoffman, 2004). Each year, an estimated 80 million cases of
P. vivax malaria develop, predominantly in Latin America, the Middle East, the Western Pacific and in Asia (
Mendis et al., 2001). In Thailand 3 decades ago,
P. vivax caused only about 20% of malaria infections and
P. falciparum 80%, but by 1998 these proportions had become approximately equal (
Chareonviriyaphap et al., 2000). Currently the standard treatment for
P. vivax malaria is a 3-day course of chloroquine in a total dose of 1,500 mg followed by primaquine, 15 mg a day for 14 days. This regimen generally produces resolution of acute symptoms and clearing of parasitemia although a substantial number of relapses subsequently develop (
Bunnag et al., 1994;
Looareesuwan et al., 1997;
Wilairatana et al., 1999). In recent years, chloroquine-resistant strains have been reported from other regions of the world (
Baird and Hoffman, 2004), but in Thailand, virtually all acute
P. vivax infections can be successfully treated with chloroquine (
Looareesuwan et al., 1999).
Elubaquine [N-(3-acetyl-4-5-dihydro-2-furanyl)-N-(6-methoxy-8-quinolinyl)1,4-pentanediamine; compound CDRI (Central Drug Research Institute) Code 80/53; bulaquine] is an 8-aminoquinoline analogue of primaquine which has shown anti-relapse activity against established sporozoite induced infections with
P. cynomolgi in rhesus monkeys (
Dutta et al., 1989;
Puri and Dutta, 1990;
Dutta et al., 1994). Initial laboratory, animal and clinical studies have suggested that elubaquine may be less toxic than primaquine. Limited studies in vitro in G6PD deficient red cells found that elubaquine produced less damage than primaquine to both normal and G6PD-deficient red cells (
Anklesaria et al., 1994). In mice, elubaquine inhibited hepatic anti-oxidant enzymes less than primaquine (
Srivastava et al., 1993). In beagle dogs, the magnitude of elubaquine-induced methemoglobinemia was 3-4 fold less than that with primaquine (
Puri et al., 1989). In patients, a comparison between primaquine and elubaquine after 7 days administration has shown that primaquine increased methemoglobin levels significantly from about 4% to 16%. By contrast, with elubaquine, the change from about 2-3% was insignificant (
Valecha et al, 2001). Elubaquine has completed Phase II/III clinical trials and is now marketed in India for use (25 mg/day for 5 days) for prevention of relapse in
P. vivax relapsed malaria (
Valecha et al., 2001;
Adak et al., 2001).
The primary objective of our study was to determine the safety and tolerability of elubaquine in comparison with primaquine, when administered for 7 days after chloroquine treatment of P. vivax. Our secondary objective was to determine if elubaquine antagonizes the activity of chloroquine against blood-stage schizonts of P. vivax. This is the first clinical study of elubaquine outside the Indian subcontinent.
PATIENTS AND METHODS
Study design
This was a randomized, open-label, prospective study of elubaquine to assess its safety and tolerability in comparison to primaquine in patients with P. vivax malaria with and without deficiency of G6PD. The study protocol was approved by the Ethics Committee of Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Study site and participants
Patients admitted to the Bangkok Hospital for Tropical Diseases between January 2004 and July 2005 were included in this study, if they were diagnosed as having malaria caused by P. vivax. P. vivax infection was defined by the presence of P. vivax asexual stage parasites on a thin blood smear. Patients enrolled in the study were 16-51 years old, weighed 40-65 kg, had the ability to take oral medications, gave informed consent, and agreed to remain in the hospital in Bangkok for a total of 28 days. The Bangkok metropolitan area has no known malaria transmission. Exclusion criteria for the study included pregnancy, lactation, concomitant infection with P. falciparum at presentation, hematocrit of < 25%, protracted vomiting, oliguria, a systolic blood pressure of < 90 mmHg, concomitant systemic disease, a history of antimalarial ingestion in previous 2 weeks, a history of allergy to primaquine or elubaquine, and a history of dark urine or significant hemoglobinuria during the course of previous malarial attack.
Interventions
All patients received 1,500 mg of chloroquine over 3 days to achieve clearance of blood-stage parasites and were then randomized to 1 of 2 treatment groups after completing chloroquine treatment, as follows: group A; primaquine 30 mg once daily for 7 days (n = 71), and group B; elubaquine 25 mg once daily for 7 days (n = 70). On the day that chloroquine therapy was completed, parasitemia clearance was documented by 2 consecutive negative results of blood smears. Primaquine and elubaquine were administered within 1 hr of a meal under direct observation. The duration of follow-up was 28 days. Out of total 141 patients, 132 (93.6%) completed 28 days' follow-up in the hospital. All patients showed negative parasitemia by 7 days after chloroquine treatment.
Procedures
Oral temperature, pulse, and respiratory rates were measured every 4 hr and blood pressure was measured once a day. Monitoring for signs and symptoms of malaria was performed daily for the first 7 days of admission and weekly thereafter. All of these patients were closely monitored for evidence of intravascular hemolysis and hemoglobinuria.
Pretreatment investigations included a complete blood count (RBC count, hemoglobin, hematocrit, total WBC count, differential count, and platelet count), serum electrolytes, total and direct bilirubin, alkaline phosphatase, blood urea nitrogen, creatinine, albumin, globulin, aspartate and alanine aminotransferases, and urinalysis. These tests were repeated on days 7, 14, 21, and 28. A screening test for G6PD deficiency was performed on admission. Thick and thin blood films were examined before treatment and every 12 hr for malaria parasites until negative, and then thick films were examined daily until discharge. In patients with G6PD deficiency, hematocrit measurements were performed on days 0 to 10, 14, 21, and 28 to detect changes in hematocrit with treatment.
Microscopic examination of blood smears was conducted using our standard operating procedure whereby 200 oil-immersion fields (magnification, x 1,000) are read on Field's stained thick blood smears. The identification of ≥ 1 asexual P. vivax parasite was recorded as a positive smear result. Blood films were considered negative if no parasites were seen in 200 oil-immersion fields in a thick blood film.
Fever clearance time (FCT) was defined as the time from the start of treatment until the oral temperature decreased to 37.0°C and remained below this for the next 48 hr. Parasite clearance time (PCT) was defined as the time from the start of treatment until the blood film was negative and remained negative for the next 24 hr.
Outcome measurements
Safety and tolerability. Patients were assessed daily during chloroquine and anti-relapse treatments. Assessments included a review of symptoms for adverse events, a clinical evaluation and a blood smear examination for malarial parasites. After completion of antimalarial drug therapy, evaluations were conducted each week to day 28. A complete blood count was determined and standard hepatic and renal function tests were conducted on admission, days 7, 14, 21 and 28. Reported adverse events in the treatment groups were compared in terms of the proportion of all randomized subjects with each adverse event.
Patients with reappearance of parasitemia after therapy with either regimen were treated with standard regimens in our hospital of chloroquine (30 mg/kg) and primaquine (15 mg a day for 14 days in patients with normal G6PD), or of chloroquine (30 mg/kg) and primaquine (30 mg once a week for 6 weeks in patients with G6PD deficiency).
Efficacy. The primary efficacy end point in our study was the efficacy of chloroquine against acute attacks of
P. vivax malaria when combined with primaquine or elubaquine. The response to treatment was defined according to Baird et al (
1997).
Statistical methods. Groups of patients were compared using the unpaired Student's t-tests for continuous variables with a Gaussian distribution, the Mann-Whitney test for nonparametric tests of continuous variables without a Gaussian distribution, and Fisher's exact test for proportions. All statistical tests were 2-tailed and a significance level of 0.05 was used.
RESULTS
A total of 141 patients were enrolled in this study, 71 in group A and 70 in group B. Demographic data and pretreatment characteristics are shown in
Table 1. Both groups were comparable with respect to clinical and laboratory characteristics. The majority of the patients had contracted the infection at the Thailand-Myanmar border.
Following treatment, 7 patients, i.e., 3 (4.3%) in group A and 4 (5.7%) in group B, left the hospital before completing 28 days of follow-up (dropped out) (
Table 2) due to social reasons unrelated to drug treatment or side effects. All were asymptomatic and negative for asexual forms before discharge from the hospital.
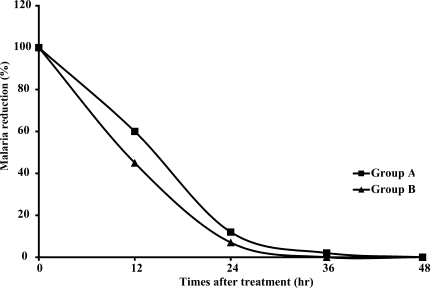
The FCT and PCT of both groups are shown in
Table 2 and
Fig. 1; there were no significant differences in either FCT or PCT. One patient with normal G6PD (1.5%) in group A had reappearance of
P. vivax on day 26 and was retreated with chloroquine (30 mg/kg) and primaquine (15 mg/day for 14 days). The difference in the cure rates at 28 days (group A: 98.5%, group B: 100%) was not significant. There was no appearance of
P. falciparum,
P. ovale, or
P. malariae in either group after treatment.
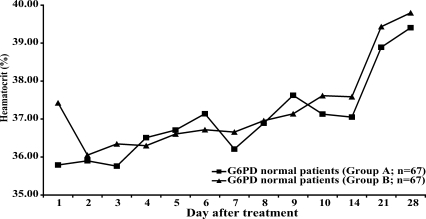
Neither group of patients with normal G6PD levels (n = 67 in each group) showed a significant reduction of hematocrit after treatment (
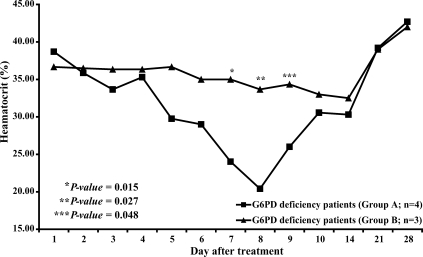
Fig. 2). By contrast, in patients with G6PD deficiency who were treated with primaquine (group A, n = 4), the mean hematocrit was significantly reduced on days 7, 8 and 9 (
P = 0.015, 0.027, and 0.048 respectively) (
Fig. 3.) compared to the mean hematocrit in patients with G6PD deficiency treated with elubaquine (group B, n = 3). Subsequently, the hematocrit in these 4 patients in group A increased without blood transfusion. No significant change in hematocrit occurred during the study in the group of patients with G6PD deficiency who were treated with elubaquine (group B, n = 3).
All adverse events listed in
Table 3 occurred during the first 3 days of administration of study drugs. These symptoms then resolved during the first week of treatment. No emesis occurred after taking the study drugs. Serious adverse effects such as cyanosis, abdominal cramp, hypertension, arrythmias, central nervous system symptoms, granulocytopenia, agranulocytosis, leucopenia, or leukocytosis were not found in patients given primaquine or elubaquine treatment. During the 28-day follow-up period, both primaquine and elubaqine were well tolerated.
DISCUSSION
The treatment of malaria caused by
P. vivax has 2 objectives: to cure acute clinical symptoms and to prevent relapses. For the first objective, chloroquine has been the standard treatment for the last 50 years. Although chloroquine-resistant strains have appeared in recent years (
Baird, 2004),
P. vivax in Thailand has remained sensitive (
Looareesuwan et al., 1999). After the 3-day regimen of chloroquine (30 mg/day) used in our study, blood levels of chloroquine remain above the minimal effective concentration for sensitive
P. vivax for as long as 35 days (
Baird, 2004). As a consequence, our study with 28 days of follow-up does not permit evaluation of elubaquine as an anti-relapse treatment for
P. vivax. Nonetheless, our results show that elubaquine does not abrogate or antagonize the antimalarial efficacy of chloroquine.
Primaquine has been used predominantly for preventing relapse originating from dormant
P. vivax hypnozoites in the liver. The conventional dose is 15 mg/day for 14 days. In an earlier study in Thailand, this regimen was associated with a 28% relapse rate during a 6-month period of observation (
Bunnag et al., 1994). A higher dose (30 mg/day for 14 days) is now recommended in patients who are not G6PD deficient (
Silachamroon et al., 2003;
Baird and Hoffman, 2004). In India, the National Anti-Malaria Program has recommended 15 mg of primaquine for only 5 days as anti-relapse treatment (
Adak et al., 2001). A recent study in India has found that this primaquine regimen (15 mg/day for 5 days) was associated with a 27% relapse rate during one year of follow-up (
Adak et al., 2001). In this same study, elubaquine (25 mg/day for 5 days) was associated with a similar overall relapse rate of about 30% at one year. Neither primaquine nor elubaquine produced a significant reduction in the overall, 1-year relapse rate, when compared to the rate of 40% observed in a group treated with placebo. In a subanalysis in this same study, a comparison of effects on relapse occurring between 7 and 12 mon after treatment found that, with respect to the rate observed in the placebo group (21%), significantly lower rates of relapse were found after treatment with either primaquine (10%) or elubaquine (14%) (
Adak et al., 2001).
In our study, to provide more comparability with previous clinical studies (
Valecha et al, 2001;
Adak et al., 2001), we used a similar dose of elubaquine, 25 mg/day, administered once daily over a 7-day period. Given our past experience in Thailand, we selected a higher dose of primaquine (30 mg/day) for study, also administered once daily over a 7-day period.
P. vivax parasitemia reappeared in only a single study patient, who had been treated with primaquine (group A), and was observed on Day 26. Because malarial transmission has not been reported in Bangkok, the reappearance of parasitemia was almost certainly not reinfection. In this study, we could not distinguish recrudescence from relapse by a chloroquine-resistant parasite (
Baird et al., 1997).
The principal potential advantage of elubaquine as an anti-relapse agent is that this drug might have less oxidative toxicity than primaquine, diminishing or eliminating methemoglobinemia and hemolysis in patients with G6PD and related erythrocytic enzyme deficiencies. Although we did not measure methemoglobin levels, and the number of patients with G6PD deficiency included in our study was small, changes in hematocrit shown in
Fig. 3 support this possibility. The 4 patients with G6PD deficiency treated with primaquine had a clinically significant fall in hematocrit, while the 3 patients with G6PD deficiency treated with elubaquine did not.
Despite the limitations of our study, the results suggest that elubaquine deserves further evaluation of both (i) safety and tolerability in patients at increased risk of oxidative toxicity, and (ii) efficacy as an anti-relapse treatment for
P. vivax malaria. Studies of safety and tolerability should focus first on individuals with well-characterized types of G6PD deficiency to determine the extent to which use elubaquine might decrease the extent of hemolysis. Studies of efficacy will need to take account of the fact that the response of malaria parasites to drugs depends not only on the species but also on the strains within the same species. Some strains have an inherent degree of drug tolerance and treatment requires a much higher dosage of an antimalarial agent than other strains. In this regard, a number of studies have shown heterogeneity in strains and geographic isolates of
P. vivax by a variability of doses of 8-aminoquinolines required to prevent relapse (
Baird and Hoffman, 2004). Thus, further studies with different
P. vivax strains and geographic isolates and with greater numbers of patients with various types of G6PD deficiency with longer periods of follow-up are needed to assess the safety, tolerability and efficacy of elubaquine in general use.
ACKNOWLEDGMENTS
The authors are grateful to the staff and nurses of the Hospital for Tropical Diseases for their help. We also thank J. Kevin Baird for his helpful comments and suggestions. This study was partly supported by Mahidol University Research Grants.
References
Fig. 1Reduction in the pertentage of malaria parasites during treatment.

Fig. 2Hematocrit in patients with normal levels of G6PD during the study period.
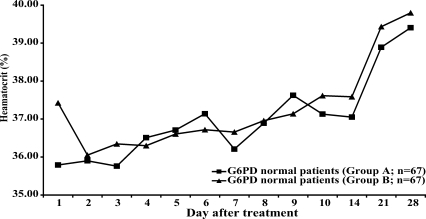
Fig. 3Hematocrit in patients with deficient levels of G6PD during the study period.

Table 1.Clinical and laboratory characteristics of study groups on admission before treatment
Table 1.
|
Group A (n = 71) |
Group B (n = 70) |
P-value |
|
Gender (Male/Female) |
53/18 |
54/16 |
0.729 |
|
Age (yr) |
|
|
|
|
Mean (±SD) |
25.03 (6.68) |
26.76 (7.98) |
0.165 |
|
Range |
16-50 |
16-51 |
|
|
Height (cm) |
|
|
|
|
Mean (±SD) |
160.80 (6.63) |
160.30 (5.38) |
0.613 |
|
Weight (kg) |
|
|
|
|
Mean (±SD) |
53.50 (6.59) |
52.95 (6.47) |
0.617 |
|
Fever [mean (±SD)] |
|
|
|
|
Highest fever before treatment (°C) |
37.59 (0.97) |
37.48 (0.84) |
0.447 |
|
No. of patients with (%) |
|
|
|
|
Splenomegaly |
3 (4.2) |
1 (1.4) |
0.317 |
|
Hepatomegaly |
16 (22.5) |
14 (20.0) |
0.713 |
|
G6PD deficiency |
4 (5.6) |
3 (4.3) |
0.713 |
|
Geometric mean parasites count (/μl) |
4,786 |
5,245 |
0.974 |
|
Range |
23~42,640 |
118~61,160 |
|
|
Laboratory data [mean (±SD)] |
|
|
|
|
Packed cell volume (%) |
35.77 (5.77) |
37.39 (6.13) |
0.109 |
|
Platelet count (/μl) |
109.96 (82.94) |
93.20 (56.33) |
0.163 |
|
White blood cell (x 103/μl) |
6.14 (1.92) |
5.83 (2.04) |
0.335 |
|
Blood urea (mg/dl) |
15.26 (5.25) |
13.90 (5.28) |
0.127 |
|
Creatinine (mg/dl) |
0.917 (0.285) |
0.845 (0.186) |
0.079 |
|
Direct bilirubin (mg/dl) |
0.501 (0.381) |
0.554 (0.480) |
0.471 |
|
Total bilirubin (mg/dl) |
1.48 (0.84) |
1.48 (0.88) |
0.968 |
|
Albumin (g/dl) |
3.76 (0.48) |
3.92 (0.49) |
0.053 |
|
Alkaline phosphatase (U/L) |
96.23 (43.64) |
85.47 (36.29) |
0.114 |
|
Aspartate aminotransferases (U/L) |
29.76 (21.20) |
28.93 (18.06) |
0.802 |
|
Alamine aminotransferases (U/L) |
31.94 (28.17) |
32.11 (29.93) |
0.972 |
Table 2.
Table 2.
|
Group A (n = 71) |
Group B (n = 70) |
P-value |
|
No. (%) of patients withdrawal |
3 (4.3%) |
4 (5.7%) |
- |
|
(day at withdrawal) |
22, 22, 22 |
14, 14, 22, 28 |
|
|
No. (%) of patients with 28-day follow-up |
68 (95.7%) |
64 (94.3%) |
- |
|
(evaluable patients) |
|
|
|
|
No. (%) of patients with P. vivax reappearance |
1 (1.5%) |
0 |
- |
|
Day of reappearance of parasitemia |
26 |
0 |
|
|
No. (%) of patient cured at day 28 |
67/68 (98.5%) |
64/64 (100%) |
0.565 |
|
Fever clearance time (hr) |
|
|
|
|
Mean (± SD) |
18.65 (13.65) |
22.74 (17.62) |
0.125 |
|
Range |
4~70 |
4~90 |
|
|
Parasite clearance time (hr) |
|
|
|
|
Mean (± SD) |
43.68 (17.29) |
44.07 (19.02) |
0.897 |
|
Range |
14~88 |
20~106 |
|
Table 3.Adverse events during study period
Table 3.
|
Group A |
Group B |
|
Headache (%) |
4 (5.6) |
3 (4.3) |
|
Abdominal pain (%) |
1 (1.4) |
0 (0) |
|
Dizziness (%) |
6 (8.5) |
4 (8.5) |
|
Nausea without vomiting (%) |
5 (7.0) |
3 (4.3) |
|
Nausea with vomiting (%) |
2 (2.8) |
0 (0) |
|
Pruritus (%) |
1 (1.4) |
0 (0) |