Abstract
Cystatin, a cysteine protease inhibitor found in many parasites, plays important roles in immune evasion. This study analyzed the molecular characteristics of a cystatin from Fasciola hepatica (FhCystatin) and expressed recombinant FhCystatin (rFhcystatin) to investigate the immune modulatory effects on lipopolysaccharide-induced proliferation, migration, cytokine secretion, nitric oxide (NO) production, and apoptosis in mouse macrophages. The FhCystatin gene encoded 116 amino acids and contained a conserved cystatin-like domain. rFhCystatin significantly inhibited the activity of cathepsin B. rFhCystatin bound to the surface of mouse RAW264.7 cells, significantly inhibited cell proliferation and promoted apoptosis. Moreover, rFhCystatin inhibited the expression of cellular nitric oxide, interleukin-6, and tumor necrosis factor-α, and promoted the expression of transforming growth factor-β and interleukin-10. These results showed that FhCystatin played an important role in regulating the activity of mouse macrophages. Our findings provide new insights into mechanisms underlying the immune evasion and contribute to the exploration of potential targets for the development of new drug to control F. hepatica infection.
-
Key words: Fasciola hepatica, cystatin, mouse macrophage, immunomodulation
INTRODUCTION
Fascioliasis is an important zoonotic disease mainly caused by
Fasciola hepatica, The disease leads to reduce growth rates and severely affects the production of livestock products such as meat, wool and milk from infected animals, posing a serious threat to animal industry [
1]. In addition,
F. hepatica is an important foodborne parasite that infects humans through raw vegetables and water contaminated with metacercariae [
2]. To date, it is estimated that at least 2.4 million people are infected and 200 million of people are at risk [
3]. Hence, fascioliasis is recognized as an important foodborne neglected tropical disease (NTD) by World Health Organization (WHO) [
4].
When encysted metacercariae enter host’s intestine, the newly excysted juveniles (NEJ) can rapidly pass through the gut wall and migrate to the liver. To cross host’s physical barriers, NEJ can secrete excretory-secretory products (ESPs) such as cathepsin L-like and B-like cysteine proteases, cystatins, and Kunitz-type inhibitors, which contribute to the parasite’s tissue invasion and evasion from the host immune responses [
5,
6]. Among them, immunomodulation of host cells is a survival strategy often adopted by the parasite. In order to survive in host for a long time,
F. hepatica can employ ESPs to induce cell apoptosis and degrade host immune molecules, thereby suppressing the activity of immune cells [
7–
9]. Cystatin is one of cysteine protease inhibitors with the activity of papain-like protein [
10]. As a virulence factor, cystatin is proposed to be involved in regulating the humoral and cellular immune responses during the infection of parasites. Currently, it has been proved that parasite cystatins can act on a variety of cells, among which macrophages are one of its target cells [
11].
Although cystatin has been identified in several parasites, the molecular characteristics and mechanism of its immune evasion have not been fully elucidated to date. With the in-depth study of
F. hepatica genome and proteomics, more proteins with immunomodulatory effects have been identified. Several studies have shown that some proteins (e.g.,
F. hepatica Stefins-1,
F. hepatica Stefins-2 and
F. hepatica Stefins-3) in
F. hepatica ESPs can inhibit mammalian cathepsin, displaying regulatory effects on host immune cells [
12]. As a potent immunomodulatory molecule in
F. hepatica, the roles of FhCystatin in host’s immune responses have not been explored in depth. Here, the molecular characteristics and enzymatic activity of a FhCystatin were characterized. Its regulatory roles on macrophage function were explored, which may provide new insights into the molecular mechanism of immune evasion exploited by
F. hepatica.
MATERIALS AND METHODS
Ethical approval
All experiments were carried out in accordance with the guidelines issued by the Ethical Committee of Shihezi University (No. A20180126).
Parasites, animals, and cells
The worms of F. hepatica were collected from naturally infected sheep in Xinjiang Uygur Autonomous Region, China. After morphological identification, the species identity was confirmed as F. hepatica based on PCR and sequencing of the second internal transcribed spacer (ITS2) of ribosomal DNA. The parasites were stored at −80°C. Six-week-old BALB/c mice were purchased from the Experimental Animal Center of Shihezi University. Mouse macrophages RAW264.7 was cultured in DMEM (Gibco, New York, New York, USA) medium supplemented with 10% fetal bovine serum (Gibco) at 37°C incubator with 5% CO2.
Primer design
Based on the genomic and transcriptomic database of F. hepatica (AY647146.1), primers were designed to amplify the FhCystatin gene, forward primer: (5′-CCGGAATTCATGTTGCGCATACTACTTG-3′) and reverse primer: (5′-GCGGCCGCTCAAGTGCAGGATACCCGAGTC-3′). For the expression of FhCystatin protein, the restriction sites EcoR I and Not I were introduced in the upstream and downstream primers, respectively.
Analysis of molecular characterization of FhCystatin protein
The total RNA of
F. hepatica was extracted using Trizol (Sigma, St. Louis, Missouri, USA), and the RNA was reverse transcribed into cDNA using a reverse transcription kit (Takara, Nojihigashi, Japan), and the cDNA was used as a template to amplify the
FhCystatin gene. The amplified product was cloned into pMD19-T vector (Takara) for sequencing. The molecular characteristic of deduced amino acid sequence, such as molecular weight, isoelectric point, signal peptide, and transmembrane region, were analyzed using the online software ProParam (
http://web.expasy.org/protparam/), ProtScale (
http://expasy.org/tools/protscale.htmL), Signal P (
http://www.cbs.dtu.dk/services/SignalP/), and TMHMM (
http://www.cbs.dtu.dk/services/TMHMM/), respectively. Sequence comparison was performed using Clustal software and the 3D structure of the FhCystatin protein was predicted by SWISS-MODEL (
https://www.swissmodel.expasy.org/).
The FhCystatin gene was subcloned into the pPIC9K vector (Invitrogen, San Diego, California, USA) to generate the pPIC9K-FhCystatin recombinant plasmid, and positive clones were screened using PCR and restriction digestion. Then, pPIC9K-FhCystatin was linearized with Sal I and electrotransformed into P. pastoris GS115 strain (Invitrogen) to screen the recombinant yeast strain. The recombinant yeast was cultured in BMGY medium and continuously induced with methanol (1%) for 5 day. Subsequently, the cells were precipitated by centrifugation and the culture supernatant was collected for SDS-PAGE analysis. Homogeneity of rFhCystatin was examined by 15% reducing SDS-PAGE. Western blot analysis was performed using Fh-infected sheep serum as primary antibody (dilution ratio 1:1,000) and horseradish peroxidase-conjugated rabbit anti-sheep IgG (Abcam, Cambridge, UK) as secondary antibody (dilution ratio 1:3,000). rFhCystatin was purified using Ni-NTA chromatography (Merck, Darmstadt, Germany) according to the manufacturer’s instructions.
Determination of the biological activity of rFhCystatin
Cathepsin B (Solarbio, Beijing, China) and Z-Arg-Arg-AMC (Sigma) were used as the target enzyme and substrate for the analysis of rFhCystatin protein activity, respectively. Briefly, cathepsin B (20 ng/reaction) was activated in buffer (0.1 M sodium acetate, 1 mM DTT, and 2 mM EDTA) at room temperature for 5 min. Then, rFhCystatin (ranging from 1 to 8 μM) was added and pre-incubated at room temperature for 20 min. Subsequently, the substrate Z-Arg-Arg-AMC was added and incubated at 37°C for 30 min. The relative activity (IC50 value) was calculated by detecting the fluorescence released from the hydrolysis of the substrate at 355 nm and 460 nm, respectively, using a multifunctional enzyme marker (Synergy2, Biotek, Burlington, Vermont, USA). In addition, rFhCystatin was incubated with buffers at different temperatures (25, 30, 37, 42, 45, and 50°C) and different pH (4–8) for 20 min, and the fluorescence values were detected to determine the optimum temperature and pH for rFhCystatin activity.
Interaction between rFhCystatin and RAW264.7 cells
Mouse RAW264.7 cells (105 cells per ml) were cultured and inoculated into confocal culture dishes (Solarbio). After the incubation with rFhCystatin (20 μg/ml) at 37°C for 2 h, these cells were fixed in 4% paraformaldehyde for 30 min at room temperature and washed 3 times with PBS (5 min/time). Then, cells were incubated with mouse anti-FhCystatin antibody (dilution 1:200) at 4°C for 12 h. Cells were washed 3 times with PBS and incubated with Cy3-conjugated goat anti-mouse IgG (Sigma) as secondary antibody (dilution 1: 500) at 37°C for 1 h. After that, cells were incubated with Hoechst 33342 (Sigma-Aldrich, St. Louis, Missouri, USA) to stain cell nuclei and observed using a laser confocal microscope (Nikon C2, Nikon, Tokyo, Japan).
Effects of rFhCystatin on macrophage proliferation and migration
The effects of rFhCystatin on the viability of RAW264.7 cells were detected using Cell counting Kit-8 (Beyotime, Haimen, China). Mouse RAW264.7 cells (10
5 cells per ml) were inoculated into 96-well tissue culture plates and incubated with different concentrations (1.0, 2.5, and 5.0 μg/ml) of rFhCystatin at 37°C for 48 h. After incubation, 10 μl of CCK-8 reagent was added to each well and incubated for 4 h protected from light. The cell survival index was calculated according to the OD values. Furthermore, the migratory roles of rFhCystatin on RAW 264.7 cells were determined as previously described [
13].
Mouse RAW264.7 cells (106 cells per ml) were cultured on 12-well plates and subsequently co-treated with different doses of rFhCystatin (1.0, 2.5, and 5.0 μg/ml), lipopolysaccharide (LPS) (1 μg/ml) and PBS was used alone as control. After 24 h, the NO content in the cells was measured using a Nitric Oxide Assay Kit (mLbio, Shanghai, China). The concentrations of interleukin-6 (IL-6), interleukin-10 (IL-10), tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β) cytokines in the supernatant of RAW264.7 cell culture were measured by enzyme-linked immunoassay kit (Merck).
Effects of rFhCystatin on apoptosis in macrophages
RAW264.7 cells were cultured on 6-well plates at a density of 106 cells/ml. Cells were treated with different concentrations of rFhCystatin (1.0, 2.5, and 5.0 μg/ml) for 24 h in a 37°C incubator with 5% CO2, and then treated with Annexin V-EGFP/PI Apoptosis Detection Kit (Beyotime) by flow cytometry (FACSAria III, Becton Dickinson, Franklin Lakes, New Jersey, USA) to assess and quantify apoptosis rates of RAW264.7 cells.
Statistical analysis of data
All data analyses were performed using GraphPad Premier 8.0 software (GraphPad Prism, San Diego, California, USA). Statistical analysis was performed using one-way ANOVA. The data are expressed as mean±standard deviation. Differences between test groups were considered significant if P-value was less than 0.05 (*P<0.05, **P<0.01, ***P<0.001, and ****P<0.0001).
RESULTS
As shown in
Supplementary Figs. S1 and S2, the open reading frame of FhCystatin gene was 351 bp in length and encoded 116 amino acids. The deduced amino acid sequence contains a signal peptide located between amino acids 20 and 21. The N-glycosylation site and transmembrane region were not found. The sequence contained a conserved cystatin-like domain (
Fig. 1A, B), the N-terminal region of conserved glycine residues and a central Q-X-V-X-G motif (
Supplementary Fig. S3). FhCystatin consists of the α-helix (35.3%), β-fold (5.2%), and irregularly curled region (33.6%), which shared 92.2% identities with type I Cystatin (AFV53480.1) of
F. gigantica at the protein level. The phylogenetic analysis showed that Fhcystatin and
F. gigantica cystatin-1 were most closely related to each other and distantly related to cystatin from other species (
Supplementary Fig. S4).
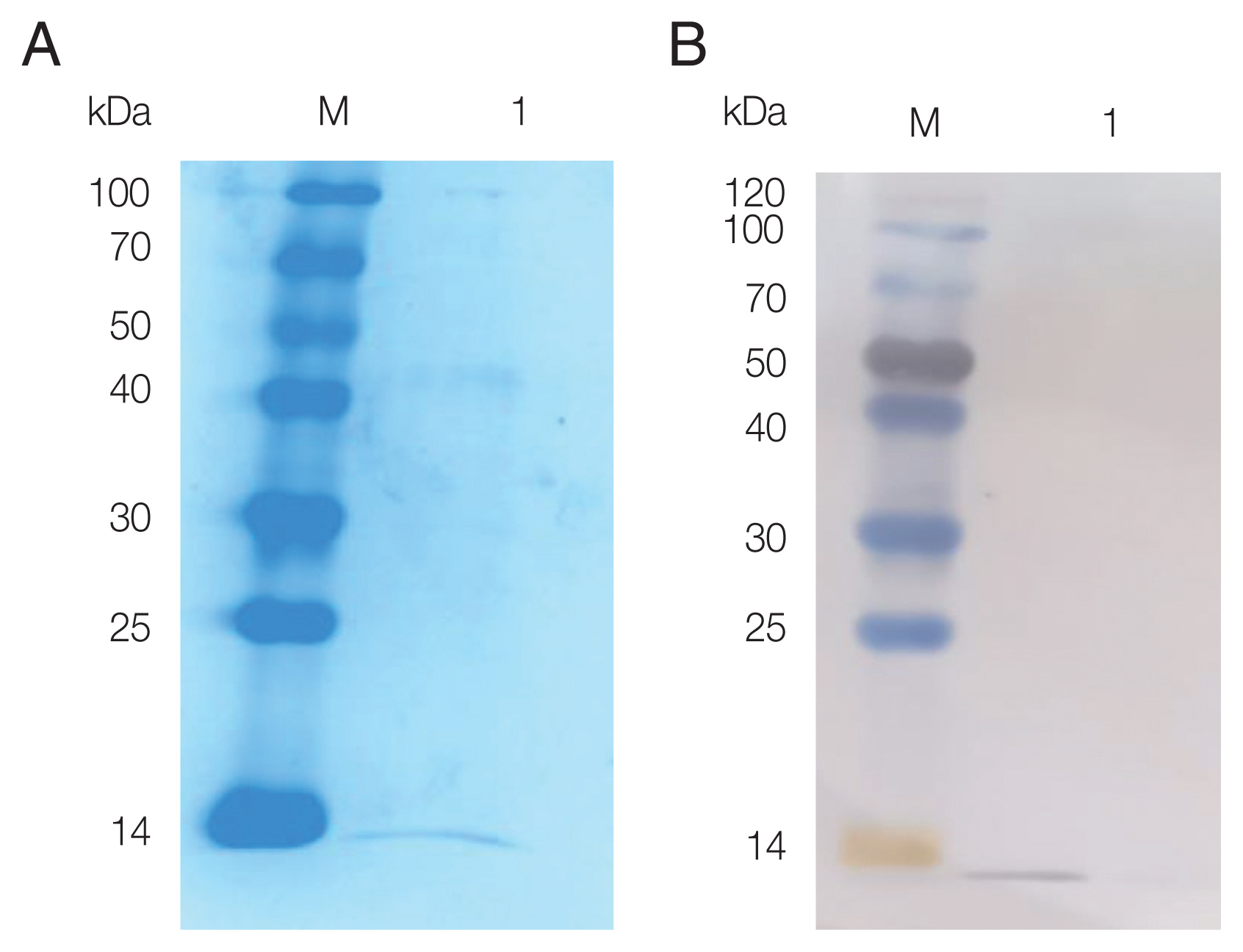
The pPIC9K-FhCystatin plasmid was constructed, and positive clones were screened using PCR and restriction digestion (
Supplementary Fig. S5). As shown in
Fig. 2, recombinant FhCystatin (rFhCystatin) was expressed in
P. pastoris, with an expected molecular weight at 12.47 kDa. Western blot confirmed that the rFhCystatin protein was recognized specifically by sheep serum infected with
F. hepatica.
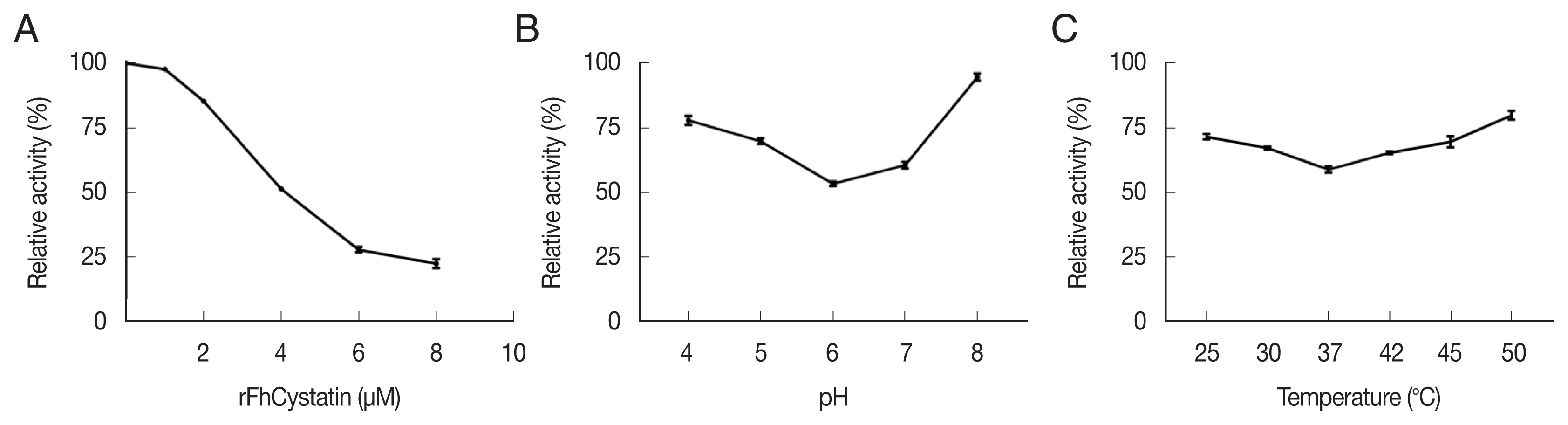
RFhCystatin inhibited cathepsin B activity in a dose-dependent manner. The inhibitory activity of rFhCystatin varied greatly at different pH and temperature; the maximum inhibitory activity was observed at pH 6 and at 37°C (
Fig. 3). As shown in
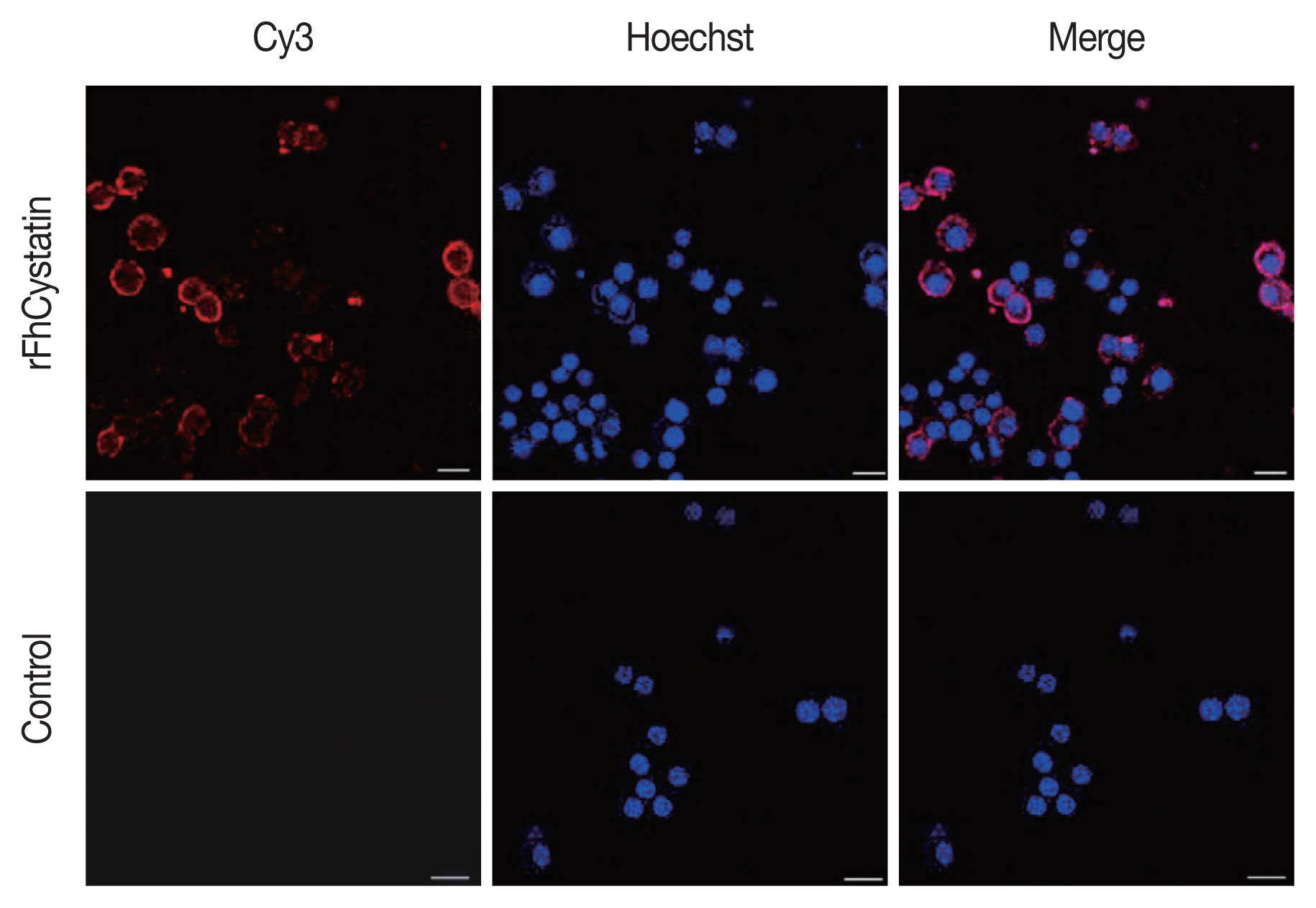
Fig. 4, rFhCystatin was bound to the surface of RAW264.7 cells. In contrast, control group failed to show the fluorescence of the red dye Cy3 on the surface of RAW264.7 cells.
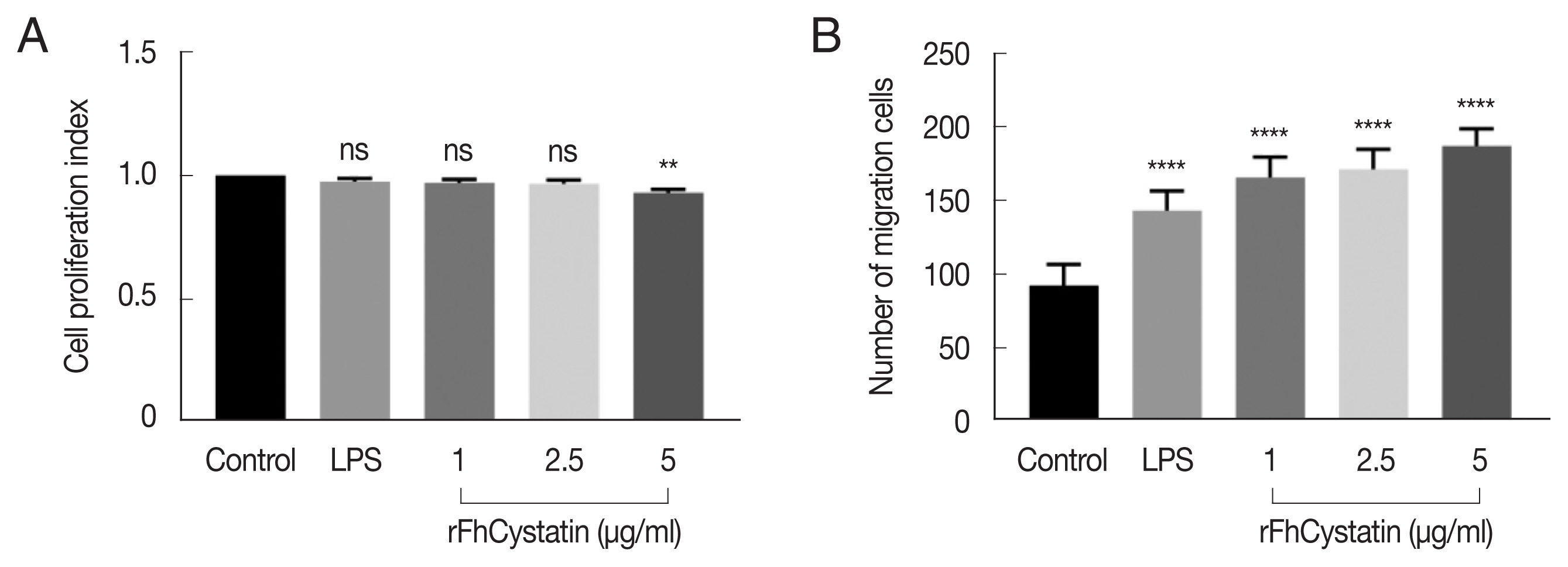
Fig. 5 demonstrated that rFhCystatin (5 μg/ml) had a significant inhibitory effect on the proliferation of RAW264.7 cells compared with PBS control and low concentration of rFhCystatin (
P<0.05). Moreover, rFhCystatin promoted the mobility of LPS-activated RAW264.7 cells (
P<0.05) and displayed a dose-dependent effect.
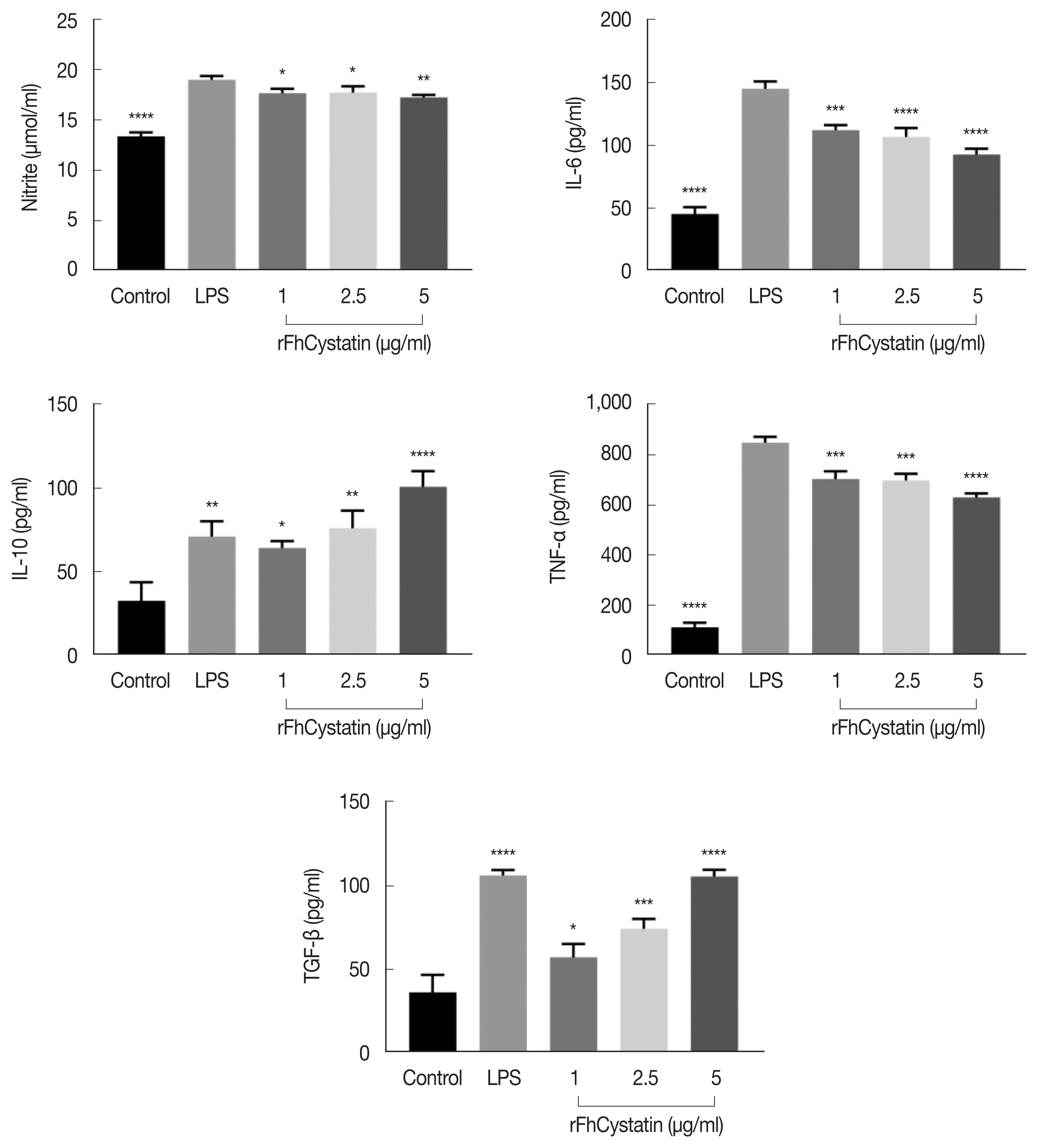
We observed effect of rFhCystatin on NO production. As shown in
Fig. 6, rFhCystatin inhibited NO production in a dose-dependent manner in RAW264.7 cells compared to positive control (LPS) (
P<0.05). Cytokine assays revealed that rFhCystatin inhibited the secretion of inflammatory factors IL-6 (
P<0.01) and TNF-α (
P<0.01) in RAW264.7 cells, while rFhCystatin significantly promoted expression of anti-inflammatory factors IL-10 (
P<0.01) and TGF-β (
P<0.01) secretion.
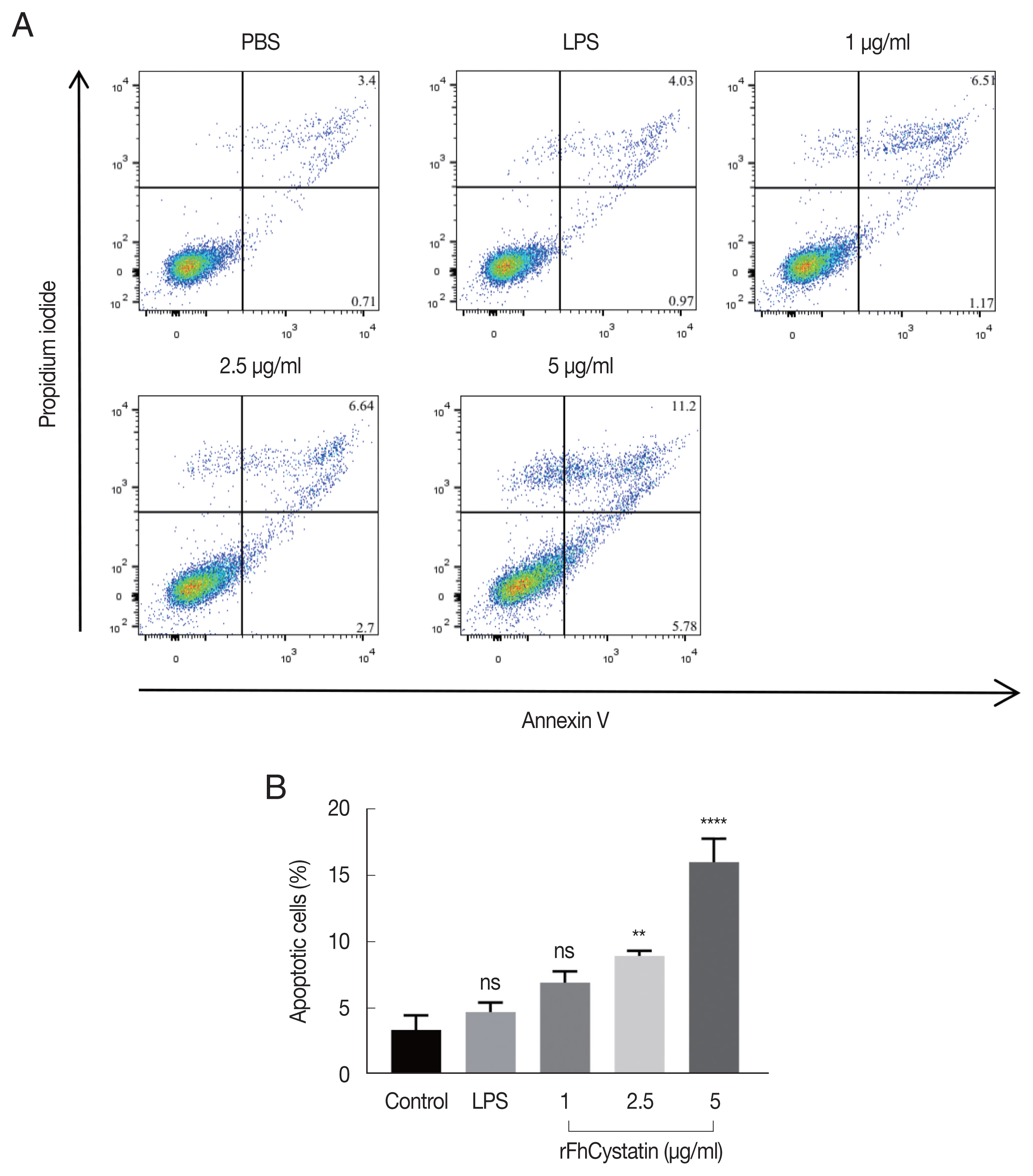
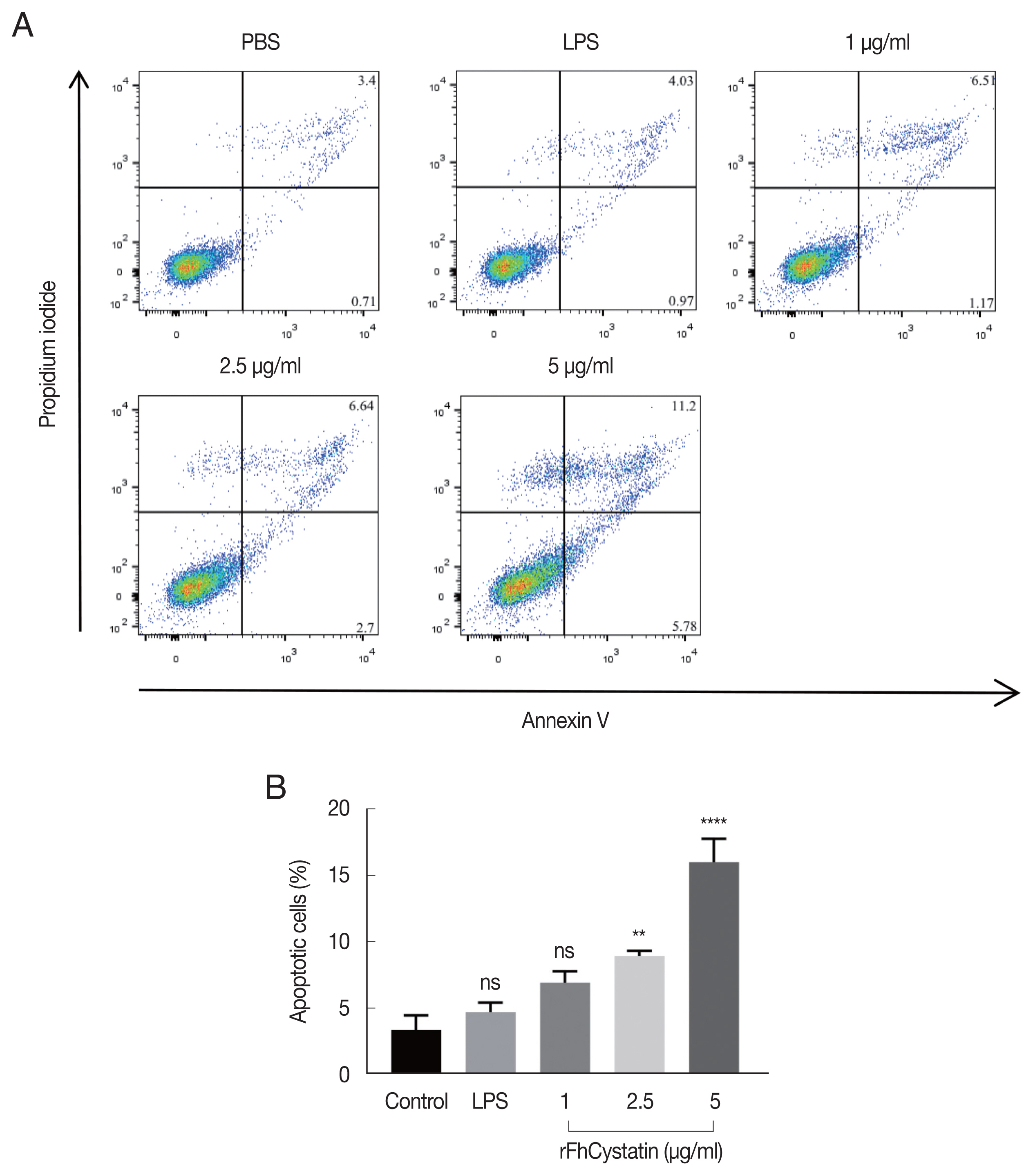
As shown in
Fig. 7, Anenxin V-FITC/PI double staining apoptosis assay revealed that rFhCystatin significantly induced apoptosis in RAW264.7 cells (
P<0.01) and showed a dose-dependent effects.
DISCUSSION
Several studies have revealed that excretory-secretory products of
F. hepatica (FhESPs) can induce apoptosis in eosinophils and peritoneal macrophages, and modify host immune responses, resulting in host immune suppression [
14]. However, the molecules mechanism of immune modulation still remains unclear, which constitutes a serious obstacle to developing protective vaccines in ruminants [
15]. A few molecules of FhESPs were known to meditate the parasite’s invasion [
16] and most of which are poorly understood in biological functions. This shortcoming has hindered the development of new drugs and vaccines against
F. hepatica infection.
Many studies have shown that immune evasion is one of the most important strategies of long-term survival in the host for parasites. Previous studies have found that parasites can achieve immune evasion through a variety of mechanisms (e.g., intracellular parasitism, antigenic mutation, and immune regulation) [
17–
19]. During parasite infection, cystatin displayed modulatory effects on host immune responses [
20]. In this study, FhCystatin gene was cloned and the molecular characteristics of encoded protein were analyzed. The results showed that
FhCystatin contained an N-terminal conserved glycine residue and a central Q-X-V-X-G motif, confirming that it is similar in molecular structure to other parasite cystatins and belongs to the cystatin family.
Several studies have revealed that cysteine protease inhibitors (cystatins) are one of the components in the ESPs of
F. hepatica [
21]. However, to date, the knowledge of expression profiles of different cystatins in
F. hepatica was limited. Considering that cysteine proteases are involved in cytophagy, intracellular clearance and antigen processing in the antigen-presenting cell (APC), cystatin produced by the parasite might inhibit the host’s cysteine protease activity, interfering with the function of APC, and modulate the host’s immune response [
22,
23]. In
Echinococcus weissei, cystatin was found to promote the production of IL-10 and reduce the production of NO and TNF-α in macrophages, thus exerted anti-inflammatory effects [
24]. The similar immunomodulatory effects of cystatin were also found in
Schistosoma twigginum, mouse intestinal parasitic nematodes, and
Schistosoma huaji [
21,
25,
26]. In
Schistosoma japonicum, cystatin stimulated the production of IL-10, but repressed the production of NO, IL-6, and TNF-α in macrophages, thereby played an important role while immune evasion [
17].
Macrophages are one of important phagocytic cells that play crucial roles in the process of antigen processing. Macrophages regulated other immune cells through secretion of a variety of cytokines [
27]. This study showed that FhCystatin bound to RAW264.7 cells and significantly inhibited the proliferation. Furthermore, FhCystatin suppressed the secretion of inflammatory factors IL-6 and TNF-α produced by RAW264.7 cells, while it significantly promoted the secretion of anti-inflammatory factors IL-10 and TGF-β. These findings suggested that FhCystatin exerted strong immunosuppressive effects. Therefore, it is speculated that the FhCystatin may be an important immunomodulatory molecule for
F. hepatica to achieve immune evasion. The detailed mechanism by which FhCystatin modulates host immune responses requires further study.
It has been reported that macrophages produced oxidative molecules such as NO and reactive oxygen to attack and damage the worm during the course of infection [
28]. We showed that the FhCystatin reduced NO production and the proliferation of RAW264.7 cells in a dose-dependent manner. However, the molecular mechanism by which FhCystatin protein suppresses NO production and proliferation of macrophages needs to be investigated.
Several studies have shown that parasitic ESPs induced the apoptosis of host cells [
13]. The peritoneal leukocytes in sheep infected with
F. hepatica underwent apoptosis to help larvae survive during peritoneal migration [
29]. A previous study reported that liver flukes might reduce the release of inflammatory factors by inducing apoptosis of goat peripheral blood mononuclear cells (PBMCs) [
30]. In turn, apoptotic cells could produce anti-inflammatory cytokines IL-10 and TGF-β to suppress the host immune responses [
31–
33]. Therefore, it can be inferred that macrophages apoptosis induced by FhCystatin may also be one of the survival strategies for
F. hepatica to evade host immune response.
This study for the first time demonstrated that FhCystatin inhibited not only the proliferation of mouse macrophages, reduced the production of NO, IL-6, and TNF-α, and promoted the production of IL-10 and TGF-β, but also the apoptosis of mouse macrophages. These findings suggested that FhCystatin plays important immunomodulatory effects on the immune function of macrophages. FhCystain also plays multiple roles in the immunological pathogenesis of F. hepatica, which provide new insights into the mechanisms of immune evasion in F. hepatica. Our study may contribute to the identification of potential targets for the development of new drug to control F. hepatica infection.
Notes
-
The authors declare that they have no conflict of interest.
Supplementary Information
ACKNOWLEDGMENTS
This work was supported by International Science & Technology Cooperation Program of XPCC (No. 2016AH006), Xinjiang Autonomous Region graduate innovation project (No. XJ2020 G085) and Grant from National Key Research and Development Program (No. 2017YFD0501202). We thank the staff who provided the technical assistance for this study.
Fig. 1Molecular characterization of FhCystatin of Fasciola hepatica. (A) Schematic structural composition of the main domains of FhCystatin. (B) 3D structure model of FhCystatin (SWISS-MODEL).
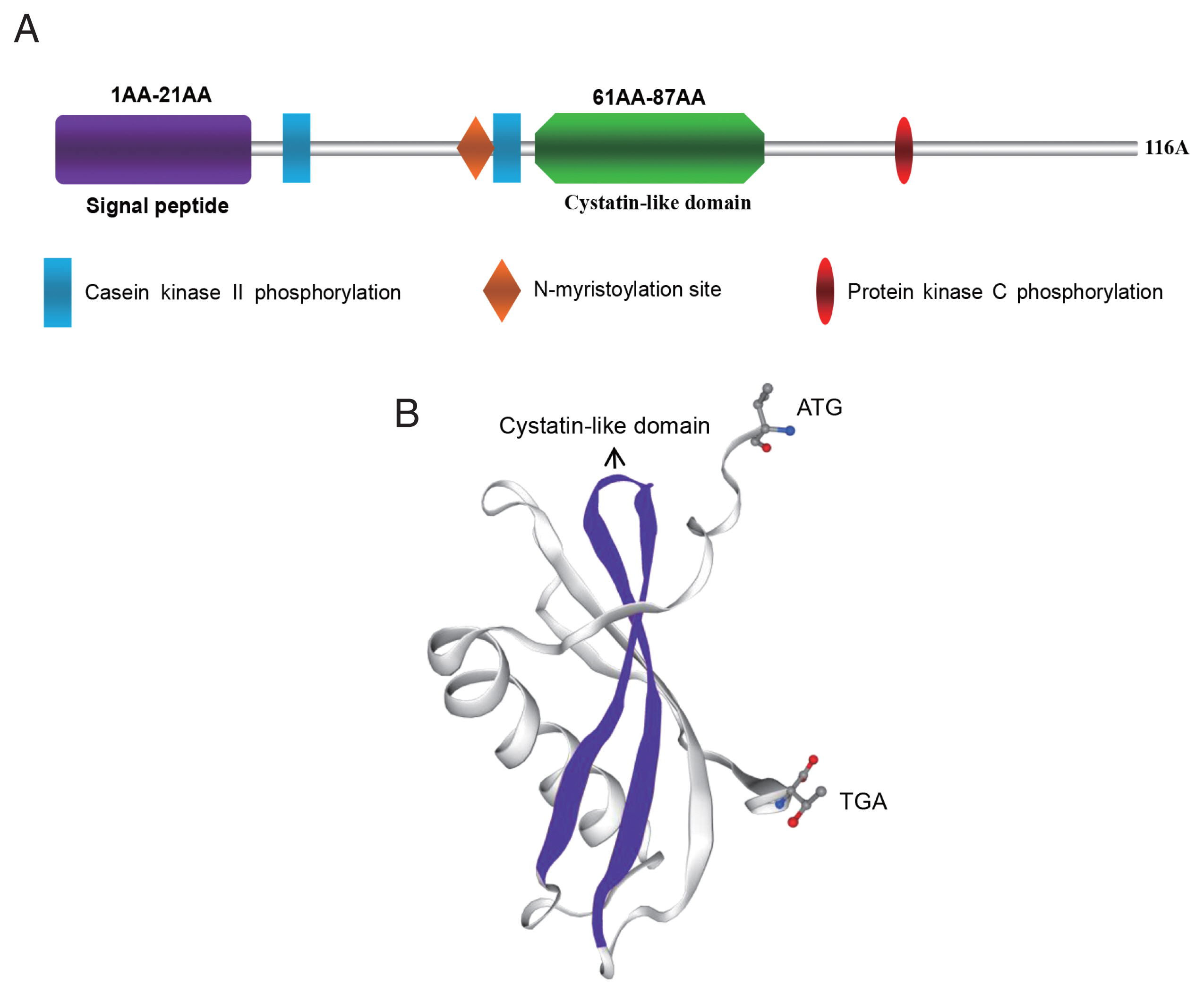
Fig. 2SDS-PAGE and western blot analysis of rFhCystatin expressed by P. pastoris. (A) Protein was resolved on 15% acrylamide gels and stained with Coomassie brilliant blue R250. Lane M: Standard molecular weight marker; Lane 1: Purified rFhCystatin. (B) The interest protein was run under non-reducing conditions, and visualized by immunodetection using specific antibodies by enhanced chemiluminescence. Lane M: Standard molecular weight marker; Lane 1: Purified rFhCystatin.
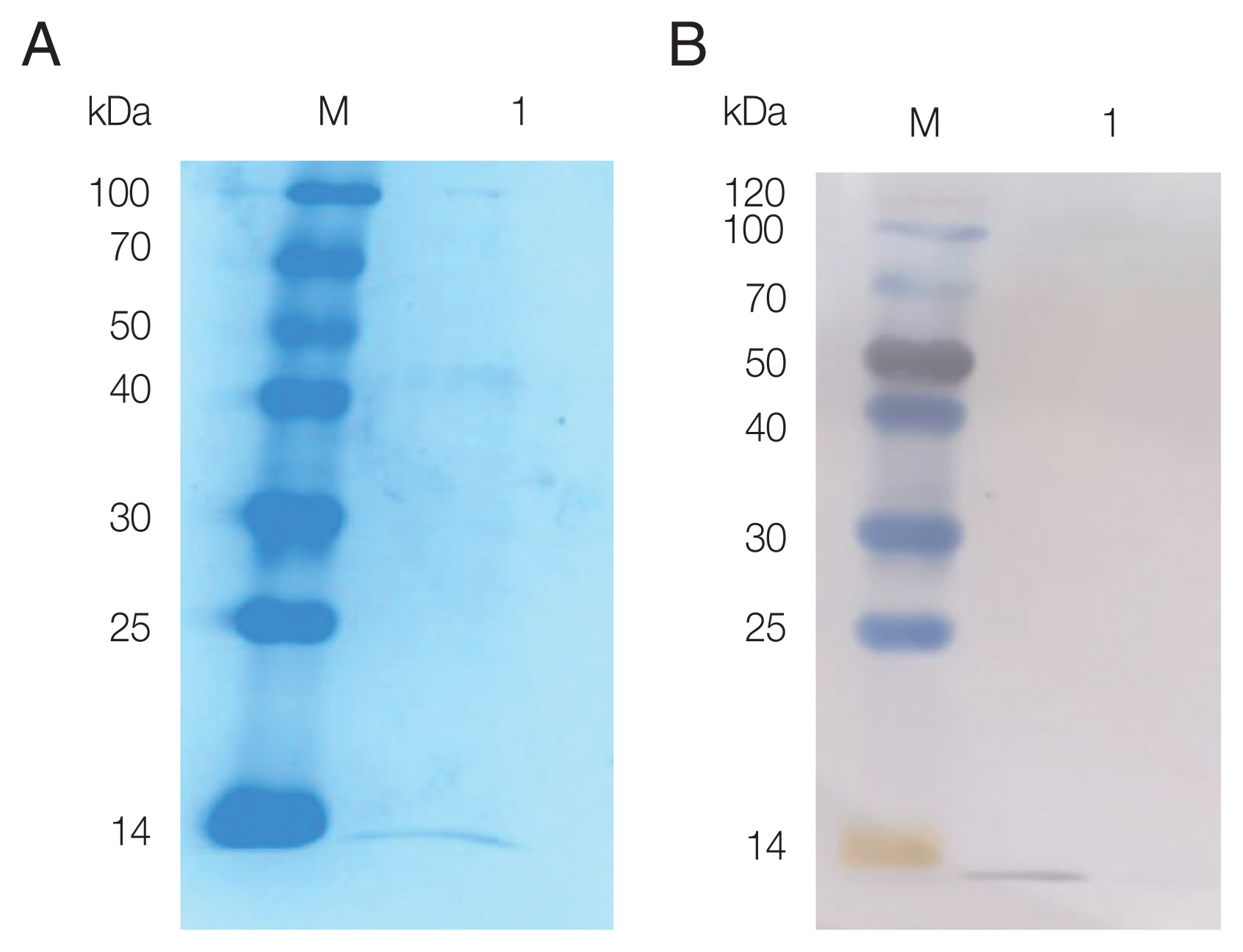
Fig. 3Determination of biological activity of rFhCystatin of Fasciola hepatica. (A) Different concentrations of rFhCystatin were incubated with cysteine proteases at room temperature for 20 min, after which residual enzyme activity was assayed. (B) rFhCystatin was incubated with cysteine proteases in different pH for 20 min at room temperature, after which residual enzyme activity was assayed. (C) rFhCystatin was incubated with cysteine proteases at different temperatures for 20 min at indicated, after which residual enzyme activity was assayed.
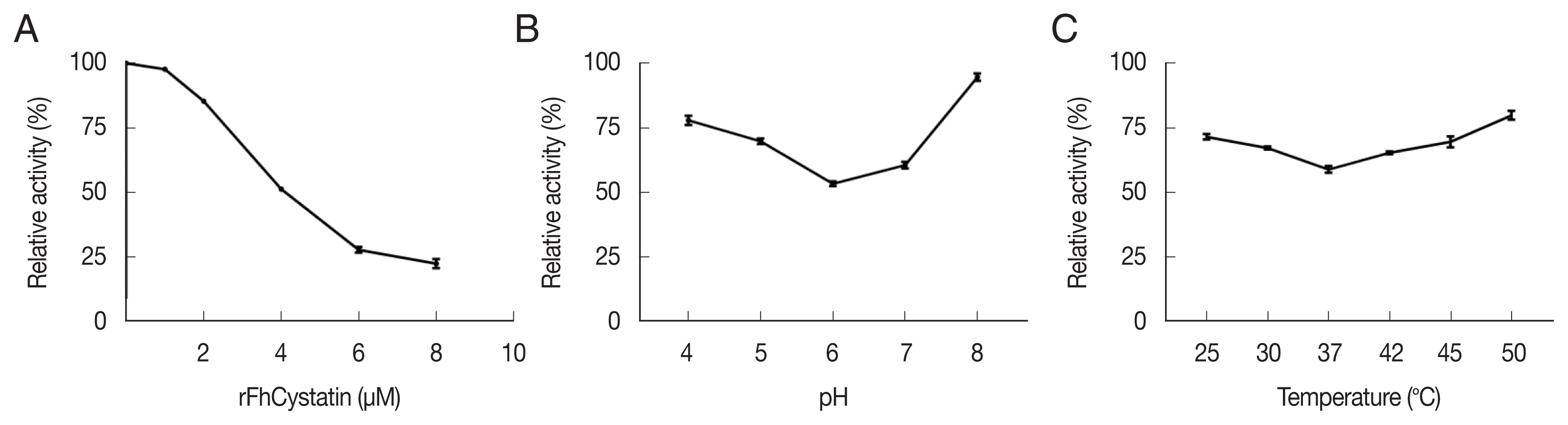
Fig. 4
Fasciola hepatica-derived rFhCystatin bound to the surface of Raw264.7 cells. Visualization of rFhCystatin protein attachment to Raw264.7 cells surface was carried out by incubation of Raw264.7 cells treated or untreated with rFhCystatin with mouse anti-rFhCystatin antibody. Hoechst (blue) and Cy3-conjugated secondary antibody (red) were used to stain host cell nuclei and rFhCystatin, respectively. Surface staining was detected in rFhCystatin-treated cells. No staining was detectable in untreated cells. Scale-bars=15 μm.
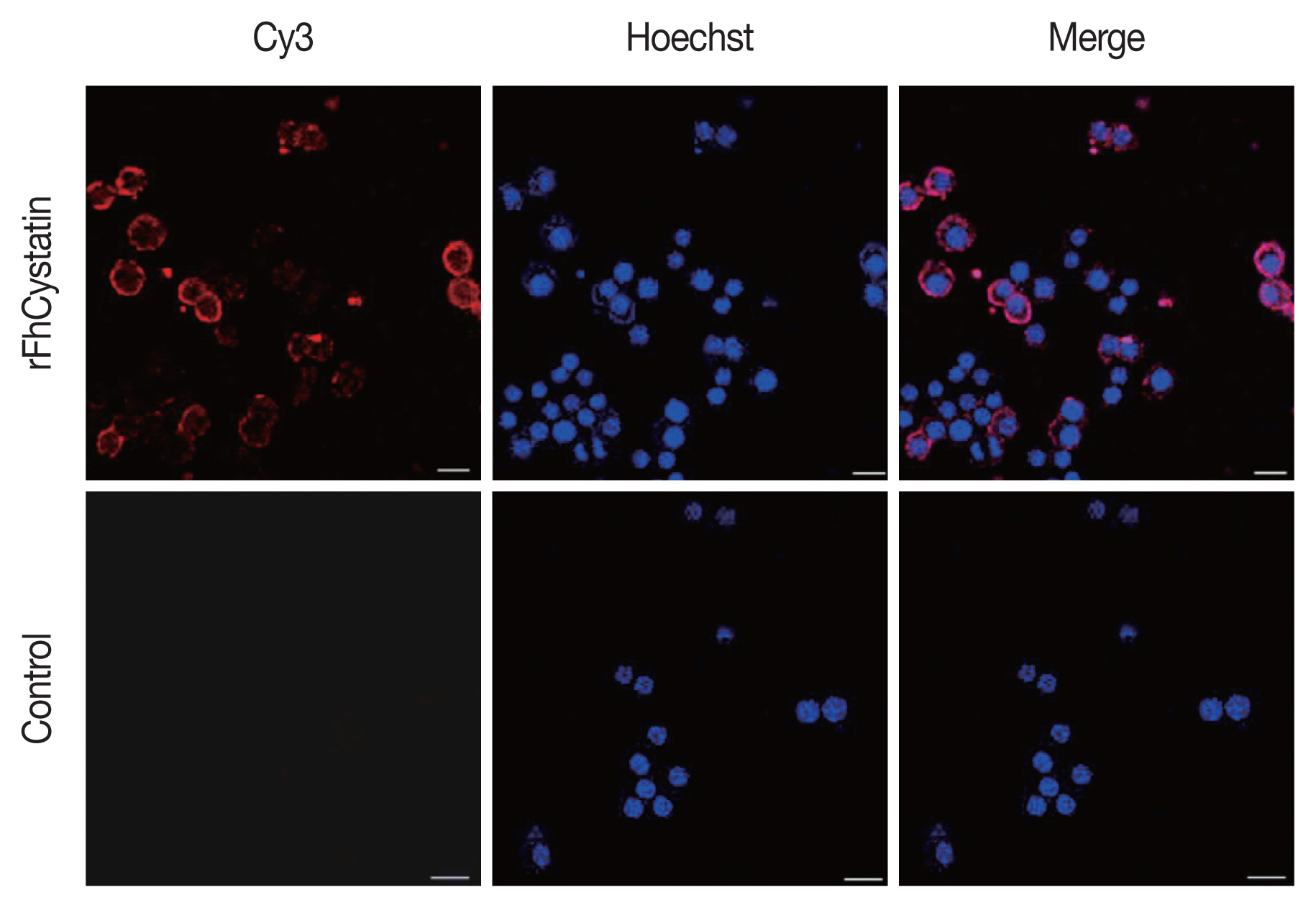
Fig. 5The effects of rFhCystatin on the proliferation and migration of RAW264.7 cells. (A) rFhCystatin inhibited RAW264.7 cell proliferation. RAW264.7 cells were sham-treated with control buffer or with different concentrations of rFhCystatin. Proliferation was determined using CCK-8 assay. (B) rFhCystatin inhibited RAW264.7 cell migration. Cells were sham-treated with control buffer or with different doses of rFhCystatin. The cell migration number was determined. Graphs represent the means±standard deviations of data from 3 independent biological replicates. ns, not-significant. **P<0.01, ****P<0.0001.
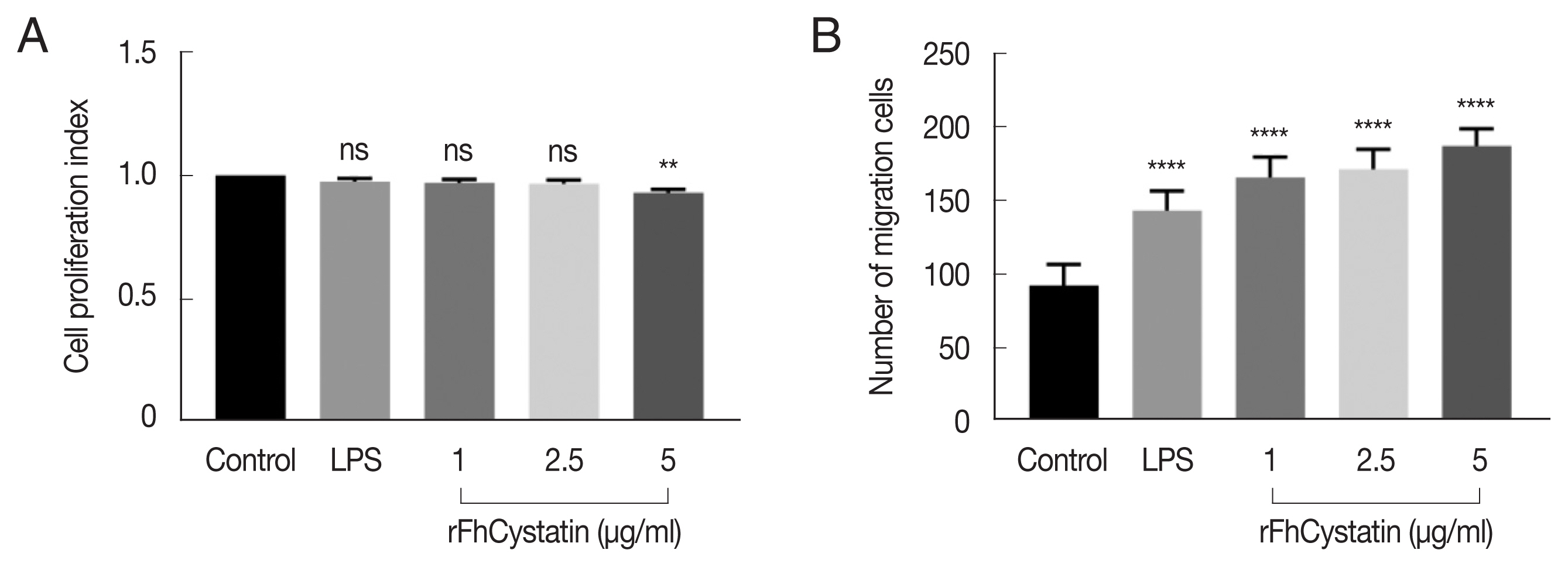
Fig. 6The effects of rFhCystatin on NO and cytokine production in RAW264.7 cells. RAW264.7 cells were incubated for 24 h in the presence or absence of different concentrations of rFhCystatin. The levels of NO and cytokine concentration in the supernatant of cultured cells were quantified by ELISA. Graphs represent the means±standard deviations of data from 3 independent biological replicates. ns, not-significant. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001.
Fig. 7rFhCystatin induced apoptosis of RAW264.7 cells. Apoptotic cells were determined by Annexin V/PI staining with flow cytometry analysis. (A) Dot plot showing death of RAW264.7 cells in response to exposure to rFhCystatin. (B) Apoptotic cells (Annexin V+/PI−) were plotted and compared with percentage of cell population. Graphs represent the means±standard deviations of data from 3 independent biological replicates. ns, not-significant. **P<0.001, ****P<0.0001.
References
- 1. Mazeri S, Rydevik G, Handel I, Bronsvoort BMD, Sargison N. Estimation of the impact of Fasciola hepatica infection on time taken for UK beef cattle to reach slaughter weight. Sci Rep 2017;7:7319. https://doi.org/10.1038/s41598-017-07396-1
- 2. Beesley NJ, Caminade C, Charlier J, Flynn RJ, Hodgkinson JE, Martinez-Moreno A, Martinez-Valladares M, Perez J, Rinaldi L, Williams DJL. Fasciola and fasciolosis in ruminants in Europe: identifying research needs. Transbound Emerg Dis 2018;65:199-216. https://doi.org/10.1111/tbed.12682
- 3. Mas-Coma S, Bargues MD, Valero MA. Human fascioliasis infection sources, their diversity, incidence factors, analytical methods and prevention measures-CORRIGENDUM. Parasitology 2020;147:601. https://doi.org/10.1017/S0031182020000256
- 4. Monica CB, Francisco A, Joachim R, Antonio S. Commentary: human liver flukes. Front Public Health 2018;30(6):122. https://doi.org/10.3389/fpubh.2018.00122
- 5. Robinson MW, Dalton JP, O’Brien BA, Donnelly S. Fasciola hepatica: the therapeutic potential of a worm secretome. Int J Parasitol 2013;43:283-291. https://doi.org/10.1016/j.ijpara.2012.11.004
- 6. Jefferies JR, Campbell AM, van Rossum AJ, Barrett J, Brophy PM. Proteomic analysis of Fasciola hepatica excretory-secretory products. Proteomics 2001;1:1128-1132. https://doi.org/10.1002/1615-9861(200109)1:9<1128::AID-PROT1128>3.0.CO;2-0
- 7. Rodríguez E, Carasi P, Frigerio S, Costa V, Vliet S, Noya V. Fasciola hepatica immune regulates CD11c+ cells by interacting with the macrophage Gal/GalNAc lectin. Front Immunol 2017;8:264. https://doi.org/10.3389/fimmu.2017.00264
- 8. Maizels RM, Smits HH, McSorley HJ. Modulation of host immunity by helminths: the expanding repertoire of parasite effector molecules. Immunity 2018;49:801-818. https://doi.org/10.1016/j.immuni.2018.10.016
- 9. Robinson MW, Donnelly S, Hutchinson AT, To J, Taylor NL, Norton RS, Perugini MA, Dalton JP. A family of helminth molecules that modulate innate cell responses via molecular mimicry of host antimicrobial peptides. PLoS Pathog 2011;7:e1002042. https://doi.org/10.1371/journal.ppat.1002042
- 10. Abrahamson M, Alvarez-Fernandez M, Nathanson CM. Cystatins. Methods Enzymol 2003;70:179-199. https://doi.org/10.1042/bss0700179
- 11. Murray J, Manoury B, Balic A, Watts C, Maizels RM. Bm-CPI-2, a cystatin from Brugia malayi nematode parasites, differs from Caenorhabditis elegans cystatins in a specific site mediating inhibition of the antigen-processing enzyme AEP. Mol Biochem Parasitol 2005;139:197-203. https://doi.org/10.1016/j.molbiopara.2004.11.008
- 12. Cancela M, Corvo I, DA Silva E, Teichmann A, Roche L, Díaz A, Tort JF, Ferreira HB, Zaha A. Functional characterization of single-domain cystatin-like cysteine proteinase inhibitors expressed by the trematode Fasciola hepatica. Parasitology 2017;144:1695-1707. https://doi.org/10.1017/S0031182017001093
- 13. Tian AL, Lu M, Calderón-Mantilla G, Petsalaki E, Dottorini T, Tian X, Wang Y, Huang SY, Hou JL, Li X, Elsheikha HM, Zhu XQ. A recombinant Fasciola gigantica 14-3-3 epsilon protein (rFg14-3-3e) modulates various functions of goat peripheral blood mononuclear cells. Parasit Vectors 2018;11:152. https://doi.org/10.1186/s13071-018-2745-4
- 14. Boukli NM, Delgado B, Ricaurte M, Espino AM. Fasciola hepatica and Schistosoma mansoni: identification of common proteins by comparative proteomic analysis. J Parasitol 2011;97:852-861. https://doi.org/10.1645/GE-2495.1
- 15. McManus DP, Dalton JP. Vaccines against the zoonotic trematodes Schistosoma japonicum, Fasciola hepatica, and Fasciola gigantica. Parasitology 2006;133:suppl. 43-61. https://doi.org/10.1017/s0031182006001806
- 16. Liu Q, Huang SY, Yue DM, Wang JL, Wang Y, Li X, Zhu XQ. Proteomic analysis of Fasciola hepatica excretory and secretory products (FhESPs) involved in interacting with host PBMCs and cytokines by shotgun LC-MS/MS. Parasitol Res 2017;116:627-635. https://doi.org/10.1007/s00436-016-5327-4
- 17. Yang X, Liu J, Yue Y, Chen W, Song M, Zhan X, Wu Z. Cloning, expression and characterisation of a type II cystatin from Schistosoma japonicum, which could regulate macrophage activation. Parasitol Res 2014;113:3985-3992. https://doi.org/10.1007/s00436-014-4064-9
- 18. Kima PE. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int J Parasitol 2007;37:1087-1096. https://doi.org/10.1016/j.ijpara.2007.04.007
- 19. Sander AF, Lavstsen T, Rask TS, Lisby M, Salanti A, Fordyce SL. DNA secondary structures are associated with recombination in major Plasmodium falciparum variable surface antigen gene families. Nucleic Acids Res 2014;42:2270-2281. https://doi.org/10.1093/nar/gkt1174
- 20. Klotz C, Ziegler T, Daniłowicz-Luebert E, Hartmann S. Cystatins of parasitic organisms. Adv Exp Med Biol 2011;712:208-221. https://doi.org/10.1007/978-1-4419-8414-2_13
- 21. Wang Y, Wu L, Liu X, Wang S, Ehsan M, Yan R, Song X, Xu L, Li X. Characterization of a secreted cystatin of the parasitic nematode Haemonchus contortus and its immune-modulatory effect on goat monocytes. Parasit Vectors 2017;10:425. https://doi.org/10.1186/s13071-017-2368-1
- 22. Nakagawa TY, Rudensky AY. The role of lysosomal proteinases in MHC class II-mediated antigen processing and presentation. Immunity Rev 1999;172:121-129. https://doi.org/10.1111/j.1600-065x.1999.tb01361.x
- 23. Chen L, He BH, Hou W, He L. Cysteine protease inhibitor of Schistosoma japonicum-a parasite-derived negative immunoregulatory factor. Parasitol Res 2017;116:901-908. https://doi.org/10.1007/s00436-016-5363-0
- 24. Behrendt P, Arnold P, Brueck M, Rickert U, Lucius R, Hartmann S, Klotz C, Lucius R. A helminth protease inhibitor modulates the lipopolysaccharide-induced proinflammatory phenotype of microglia in vitro. Neuroimmunomodulation 2016;23:109-121. https://doi.org/10.1159/000444756
- 25. SW , Cho MK, Park MK, Kang SA, Na BK, Ahn SC, Kim DH, Yu HS. Parasitic helminth cystatin inhibits DSS-induced intestinal inflammation via IL-10+F4/80+ macrophage recruitment. Korean J Parastiol 2011;49:245-254. https://doi.org/10.3347/kjp.2011.49.3.245
- 26. Sun YX, Liu GY, Li ZT, Chen Y, Liu YF, Liu BY, Su Z. Modulation of dendritic cell function and immune response by cysteine protease inhibitor from murine nematode parasite Heligmosomoides polygyrus. Immunology 2013;138:370-381. https://doi.org/10.1111/imm.12049
- 27. Varin A, Gordon S. Alternative activation of macrophages: immune function and cellular biology. Immunobiology 2009;214:630-641. https://doi.org/10.1016/j.immuni.2010.05.007
- 28. Cervi L, Rossi G, Cejas H, Masih DT. Fasciola hepatica-induced immune suppression of spleen mononuclear cell proliferation: role of nitric oxide. Clin Immunol Immunopathol 1998;87:145-154. https://doi.org/10.1006/clin.1997.4499
- 29. Escamilla A, Pérez-Caballero R, Zafra R, Bautista MJ, Pacheco IL, Ruiz MT, Martínez-Cruz MS, Martínez-Moreno A, Molina-Hernández V, Pérez J. Apoptosis of peritoneal leucocytes during early stages of Fasciola hepatica infections in sheep. Vet Parasitol 2017;238:49-53. https://doi.org/10.1016/j.vetpar.2017.03.015
- 30. Chen D, Tian AL, Hou JL, Li JX, Tian X, Yuan XD, Li X, Elsheikha HM, Zhu XQ. The multitasking Fasciola gigantica Cathepsin B interferes with various functions of goat peripheral blood mononuclear cells in vitro. Front Immunol 2019;10:1707. https://doi.org/10.3389/fimmu.2019.01707
- 31. Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature 1997;390:350-351. https://doi.org/10.1038/37022
- 32. Bzowska M, Guzik K, Barczyk K, Ernst M, Flad HD, Pryjma J. Increased IL-10 production during spontaneous apoptosis of monocytes. Eur J Immunol 2002;32:2011-2020. https://doi.org/10.1002/1521-4141(200207)32:7<2011::AID-IMMU2011>3.0.CO;2-L
- 33. Chen WJ, Frank ME, Jin W, Wahl SM. TGF-β released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity 2001;14:715-725. https://doi.org/10.1016/s1074-7613(01)00147-9
 , Yucheng Liu1,†, Guowu Zhang1,†, Xifeng Wang1, Zhiyuan Li1, Yunxia Shang1, Chengcheng Ning1, Chunhui Ji1, Xuepeng Cai2, Xianzhu Xia1, Jun Qiao1, Qingling Meng1,*
, Yucheng Liu1,†, Guowu Zhang1,†, Xifeng Wang1, Zhiyuan Li1, Yunxia Shang1, Chengcheng Ning1, Chunhui Ji1, Xuepeng Cai2, Xianzhu Xia1, Jun Qiao1, Qingling Meng1,*