Abstract
The discovery of new antimalarial drugs can be developed using asynchronized Plasmodium berghei malaria parasites in vivo in mice. Studies on a particular stage are also required to assess the effectiveness and mode of action of drugs. In this report, we used endoperoxide 6-(1,2,6,7-tetraoxaspiro [7.11] nonadec-4-yl) hexan-1-ol (N-251) as a model antimalarial compound on P. chabaudi parasites. We examined the antimalarial effect of N-251 against ring-stage- and trophozoite-stage-rich P. chabaudi parasites and asynchronized P. berghei parasites using the 4-day suppressive test. The ED50 values were 27, 22, and 22 mg/kg, respectively, and the antimalarial activity of N-251 was verified in both rodent malaria parasites. To assess the stage-specific effect of N-251 in vivo, we evaluated the change of parasitemia and distribution of parasite stages using ring-stage- and trophozoite-stage-rich P. chabaudi parasites with one-day drug administration for one life cycle. We discovered that the parasitemias decreased after 13 and 9 hours post-treatment in the ring-stage- and trophozoite-stage-rich groups, respectively. Additionally, in the ring-stage-rich N-251 treated group, the ring-stage parasites hindered trophozoite parasite development. For the trophozoite-stage-rich N-251 treated group, the distribution of the trophozoite stage was maintained without a change in parasitemia until 9 hours. Because of these findings, it can be concluded that N-251 suppressed the trophozoite stage but not the ring stage. We report for the first time that N-251 specifically suppresses the trophozoite stage using P. chabaudi in mice. The results show that P. chabaudi is a reliable model for the characterization of stage-specific antimalarial effects.
-
Key words: Plasmodium chabaudi, synchronization, stage-specific activity, antimalarial N-251
Introduction
A new antimalarial drug study usually begins with the use of
Plasmodium falciparum in vitro and rodent malaria parasites such as
Plasmodium berghei and
Plasmodium chabaudi in vivo [
1,
2]. However, drug effectiveness for rodent malaria species does not always mimic those of the human malaria parasite,
P. falciparum [
3]. In mice
P. chabaudi produces a high parasitemia and generates synchronous infections by circadian rhythm, thus enabling studies on parasite stage specificity [
4,
5]. The several
Plasmodium spp. life cycle stages offer various patterns and targets for antimalarial chemotherapy [
6].
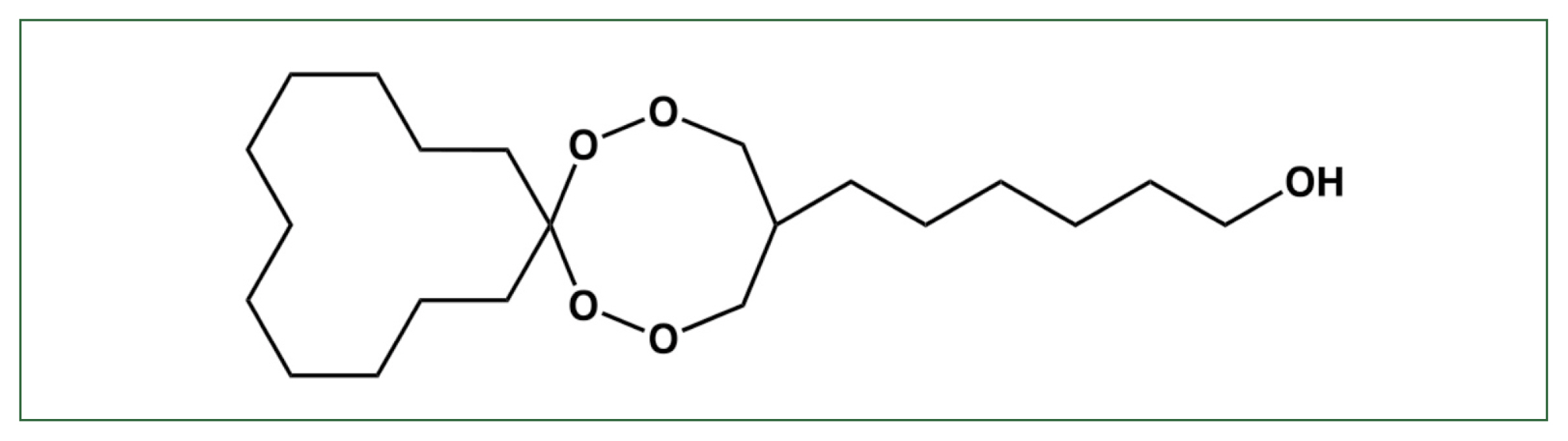
The drug candidate 6-(1,2,6,7-tetraoxaspiro [7.11] nonadec-4-yl) hexan-1-ol endoperoxide (N-251) has high antimalarial activity against both
P. falciparum in vitro and
P. berghei in vivo, as described by our previous studies [
7,
8]. We also found that N-251 inhibited the trophozoite stage exclusively on synchronized
P. falciparum with sorbitol treatment in vitro [
9]. In this research, we used N-251 as a model antimalarial compound for analyzing stage-specific inhibition using synchronized
P. chabaudi in vivo.
Melatonin is a natural hormone synthesized by the pineal gland and controlled by the circadian rhythm [
10]. Some reports found that
Plasmodium becomes more synchronized by melatonin [
11,
12]. We evaluated the ability of melatonin to obtain a high yield of synchronized
P. chabaudi parasites. Our study aimed to examine the feasibility of an animal experimental model for stage-specific antimalarial effect using N-251 on the predominant ring and trophozoite stages of
P. chabaudi.
Materials and Methods
Parasites and mice
P. chabaudi AS strain (P. chabaudi) and P. berghei NK65 strain (P. berghei) parasites were maintained in the laboratory. ICR male mice (The Jackson Laboratory, Kanagawa, Japan) were maintained in a 12 h light/dark cycle at room temperature (23±2°C) with free access to food (MF diet, Oriental Co., Tokyo, Japan) and water. The study was carried out in compliance with the Okayama University Guidelines for Animal Experimentation and was authorized by the Institutional Animal Care and Use Committee (permission number: OKU-2022815).
Comparison between parasite growth of P. chabaudi and P. berghei
To initiate growth rate assays, mice were intravenously (iv) inoculated with 1×106 P. chabaudi or P. berghei parasite-infected erythrocyte suspensions obtained from infected donor ICR mice. The parasitemia was assessed using a microscope after the preparation of a thin smear. Data were expressed as the mean (n=5) with a bar showing the standard deviation (SD) value.
Effect of melatonin
Melatonin (Sigma, St. Louis, MO, USA) was dissolved in 10.0% ethanol, 10.0% Cremophore EL, and 80.0% saline (1:1:8 [v/v/v]). P. chabaudi and P. berghei parasites were evaluated by iv infusion of 3×105 infected erythrocytes in a healthy mouse. Mice received 4 days of melatonin iv administration at a rate of 50 mg/kg/day (days 0, 1, 2, and 3). Vehicles were the only treatment for the control groups. Parasitemia and distribution of parasite stages were checked on day 4. Data were expressed as the mean (n=5) with a bar indicating the SD value.
Stage-specific effect of N-251
Ring-stage- and trophozoite-stage-rich parasite groups of
P. chabaudi were examined following iv administration of 1×10
7 infected erythrocytes in mice. When the ring and trophozoite stages reached about 60.0% of the abundance of all stages, N-251 (
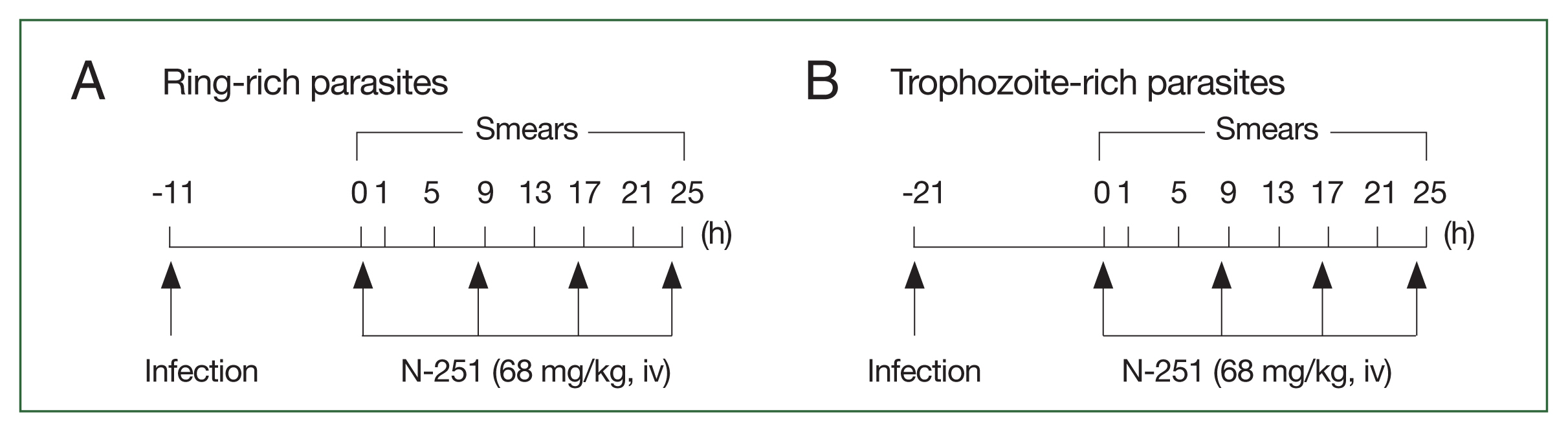
Fig. 1) (dissolved in 10.0% ethanol, 10.0% Cremophore EL, and 80.0% saline (1:1:8 [v/v/v]) was given iv at a dose of 68 mg/kg every 8 h for 1 day. Control groups were treated with vehicles only. As shown in
Fig. 2, blood smears were examined every 4 h for 25 h. Data were expressed as the mean (
n=5) with the bar indicating the SD value.
The antimalarial effect of N-251 was analyzed using the 4-day suppressive test in vivo [
13]. Synchronized
P. chabaudi and asynchronized
P. berghei parasites were tested by iv infusion of 1×10
6 infected erythrocytes in a healthy mouse. We prepared 2 synchronized
P. chabaudi groups: one in which the ring stage made up roughly 60% of the infected erythrocytes and the other in which the trophozoite stage made up more than 60% of the infected erythrocytes. Two hours post-infection, mice were iv administered N-251 at doses of 5, 20, and 50 mg/kg/day for 4 days. Control groups were treated with vehicles only. On day 4, thin blood films were prepared and the parasitemias were calculated. Data were expressed as the mean (
n=5)±SD value.
Results were examined using Microsoft Excel 2016 and the data were expressed as the mean ±SD value. Percent parasitemia was computed by dividing the number of infected RBCs by the total number of RBCs indexed and multiplied by 100. ED50 and ED90 values were collected by sigmoidal dose-response curve analysis.
Results
Comparison between parasite growth of P. chabaudi and P. berghei
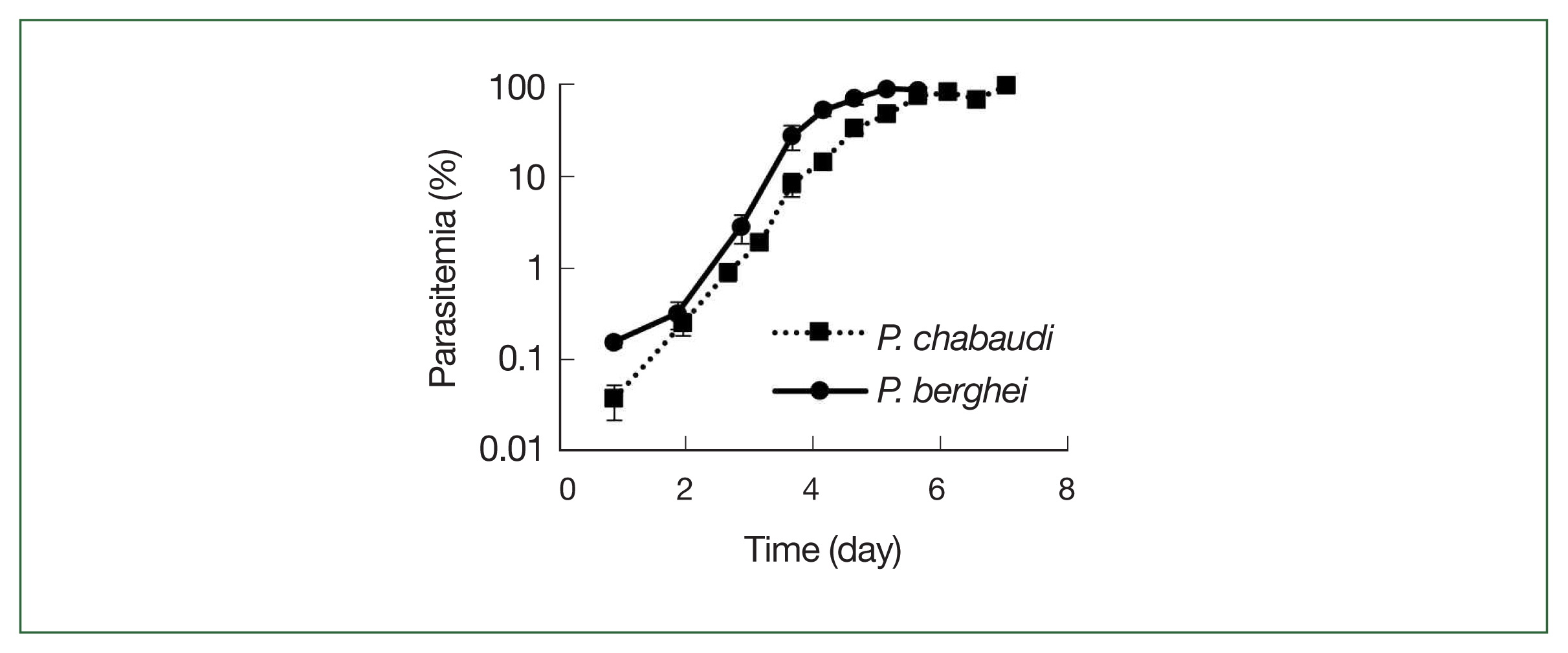
The growth rates of
P. chabaudi and
P. berghei parasites in mice are displayed in
Fig. 3. Parasite growth was more delayed in
P. chabaudi than
P. berghei, and the growth curves slope were otherwise similar for both parasite strains, and all infected mice died after day 7.
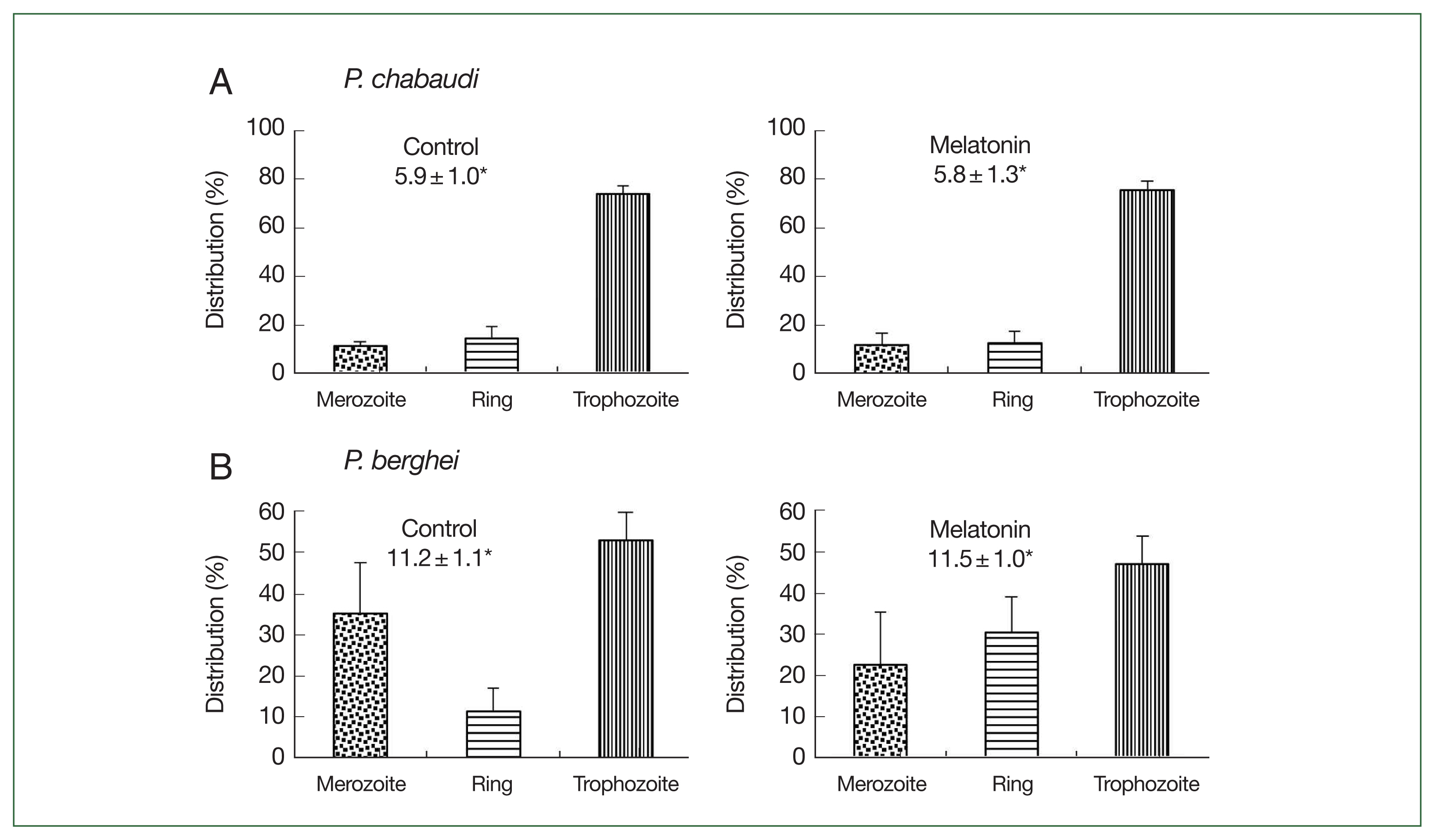
The effect of melatonin on
P. chabaudi and
P. berghei is shown in
Fig. 4. In the
P. chabaudi parasite groups, more than 70.0% of parasites were synchronized to the trophozoite stage in both the control and melatonin-treated groups. The parasitemias were 5.9% and 5.8%, respectively, in the control and melatonin-treated groups (
Fig. 4A). This data demonstrated that melatonin did not affect the synchronization and growth of
P. chabaudi. For
P. berghei parasites, the distributions of merozoites, ring, and trophozoite stages in the control group were 35.3, 11.4, and 53.1%; while in the melatonin-treated group, the distributions were 22.6, 30.4, and 46.9%, respectively, without change in parasitemia (control: 11.2%, melatonin: 11.5%) (
Fig. 4B). From these findings it was apparent that melatonin had a partial effect on the development of parasite stages in
P. berghei. As a result, we used
P. chabaudi without melatonin in the next experiment.
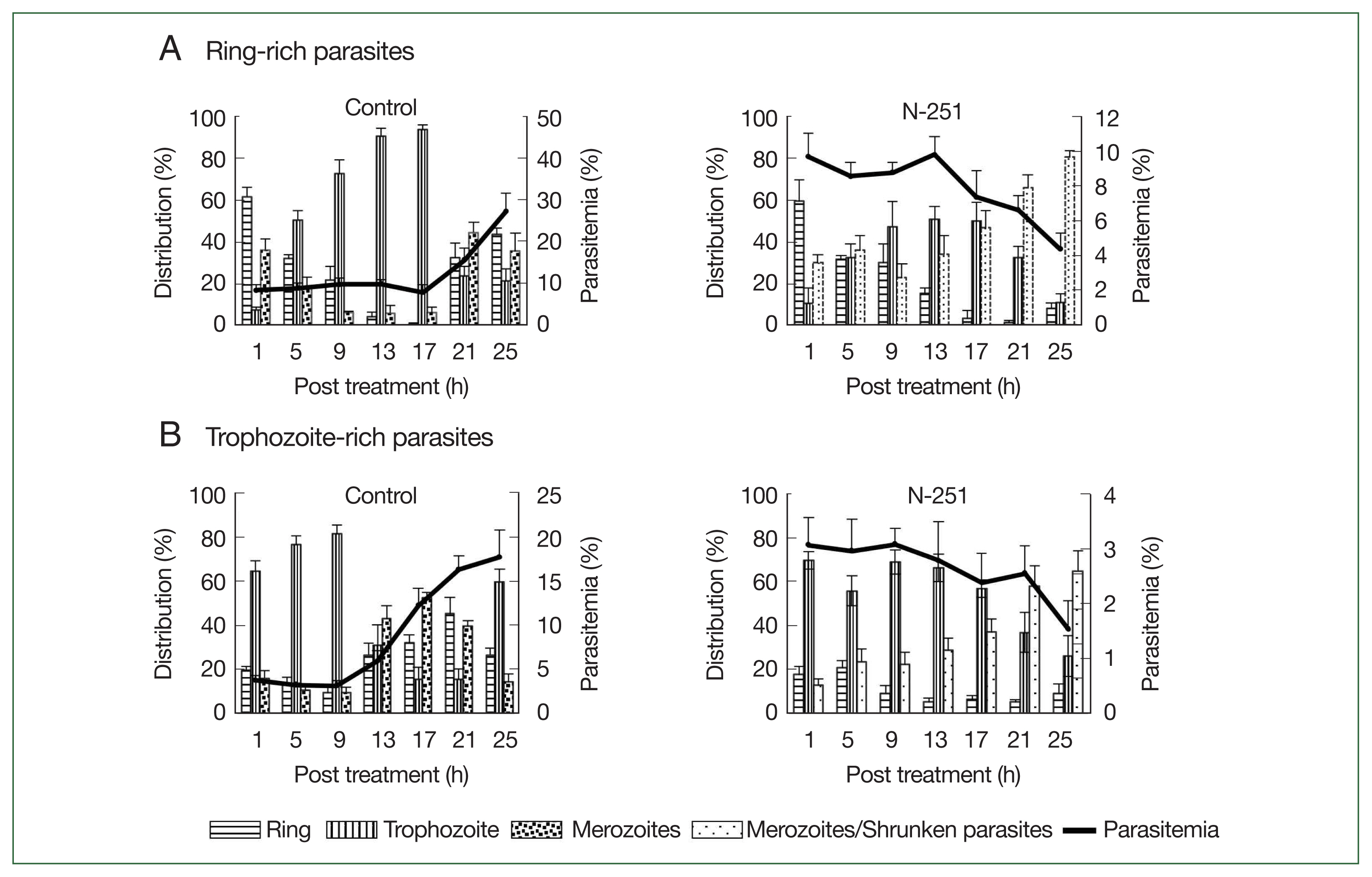
To assess the stage-specific effect of N-251 in vivo, we examined the change of parasitemias and distributions of parasite stages using preparations of ring-stage- and trophozoite-stage-rich
P. chabaudi parasites. The outcomes employing parasite preparations rich in ring stages are shown in
Fig. 5A. In the control group, the parasitemia increased after 17 h, and ring-stage parasites developed to the trophozoite stage until 17 h. The distribution of ring-stage parasites in the N-251 group reduced from 60.0 to 15.0% after 13 h, whereas trophozoite-stage parasites rose from 10.1 to 51.2% in the same period (
Fig. 5A). The effect of N-251 on trophozoite-stage-rich parasites is depicted in
Fig. 5B. The trophozoite-stage parasites made up around 81.0% of all parasites within the same parasitemia up to 9 h in the control group, after which the parasitemia dropped to 17.7% at 25 h. For the N-251 group, the parasitemias and the distribution ratio of the trophozoite stage were not changed until 9 h; and finally, the parasitemia decreased to 1.5% at 25 h. To compare ring-stage- and trophozoite-stage-rich N-251 groups, the time point of decreasing parasitemia was 4 h earlier in the trophozoite stage than in the ring-stage-rich group without marked changes in stages distributions. According to these findings, N-251 selectively suppressed the trophozoite stage and had no discernible impact on the ring stage.
We evaluated the antimalarial effect of N-251 against ring-stage- and trophozoite-stage-rich
P. chabaudi and asynchronized
P. berghei parasites (
Table 1). N-251 inhibited the growth of
P. chabaudi and
P. berghei with similar ED
50 and ED
90 values. There was no discernible difference in the ED value between
P. berghei and the various
P. chabaudi parasite stages.
Discussion
Evaluation of the stage-specific activities of antimalarial candidates in vivo is crucial for studying the effectiveness and mechanisms of action. In this study, we evaluated the stage-specific antimalarial activity of
P. chabaudi using the predominant ring and trophozoite phases. It was reported that the
P. chabaudi malaria parasite possesses an auto synchronization of intraerythrocytic developmental stages via a circadian rhythm [
14]. Additionally, according to some scientists, host melatonin synchronizes the growth of the parasite stages [
15,
16]. In a healthy human body, melatonin is typically secreted at a rate of up to 0.04 mg/kg. Melatonin has a short half-life in the blood, of less than 30 min and it is relatively non-toxic [
17–
19]. As a result, in this study, melatonin was administrated at 50 mg/kg and the effect on parasites was examined. We discovered that melatonin had no antimalarial activity or improvement of synchronization of intraerythrocytic parasite developmental stages in
P. chabaudi (
Fig. 4A). In the
P. berghei melatonin-treated group, the distribution of merozoites was decreased and ring-stage parasites increased without antimalarial activity (
Fig. 4B). These results show that melatonin had no antimalarial activity in both species of rodent malaria parasites and affects young parasite (merozoite) development of
P. berghei. Further studies are required to assess melatonin function in
P. berghei. The
P. berghei strain is not appropriate for analyzing the stage specificity of the drug in our study, similar to the reported results [
20]. With
P. chabaudi, it is plausible that the mechanism of action can be examined because the drug can act at a specific stage.
In studies of the antimalarial activity of drug candidates, including the characterization of the stage specificity of action, the drug sensitivity of given rodent malaria species does not always mimic that of
P. falciparum [
3]. We have previously investigated the generation of novel antimalarial drugs in
P. falciparum in vitro and
P. berghei in vivo employing thousands of molecules, including natural products, marine sources, and synthesized compounds [
21–
24]. From these studies, N-251 was chosen as a promising antimalarial candidate. N-251 specifically suppressed the trophozoite stage of
P. falciparum in vitro, possibly by targeting the
P. falciparum endoplasmic reticulum-resident calcium-binding protein (
PfERC) [NCBI accession no. 124803623] [
9,
25,
26]. The
PfERC level changes with parasite growth, increasing to a maximum level during the trophozoite stage and was inhibited by N-251 treatment. We examined in vivo antimalarial activity of N-251. N-251 revealed strong antimalarial activity with a cure effect in
P. berghei in mice, but the stage specificity of its action was not investigated [
8]. In the present study, the stage-specific antimalarial activity of N-251 as a model compound on
P. chabaudi in mice was studied. If we discover that N-251 specifically inhibits the trophozoite stage in vivo, then
P. chabaudi would be an experimental model for stage-specific antimalarial assessment in vivo. The parasites continued to proliferate to the trophozoite stage before being inhibited by N-251 in the group of parasites with a high concentration of ring-stage parasites (
Fig. 5). In the trophozoite-stage-rich N-251 group, the ratio of trophozoite-stage parasites was similar while merozoites and shrunken parasites were increased with the same parasitemia up to 9 h. Because we were unable to distinguish between typical merozoites and parasites with morphologically altered merozoite-shaped bodies, the results in
Fig. 5 reflected the total of merozoites and shrunken parasites. The results report for the first time that N-251 particularly inhibits the
P. chabaudi trophozoite parasite in mice. Further study is required to clarify N-251 stage-specific action using additional synchronized trophozoite parasites.
We expected higher effectiveness of N-251 could be collected using trophozoite-stage-rich P. chabaudi, but we received similar ED50 values with ring-stage- and trophozoite-stage-rich P. chabaudi and asynchronous P. berghei using the 4-day suppressive test. The 4-day suppressive test only reveals a drug’s overall impact after 4 days; it does not provide specific information on the various parasite stages. The parasite synchronization may be interrupted in the experiment since the life cycle of rodent malaria parasites is one day.
We conclude that melatonin has no antimalarial or synchronizing effect on P. chabaudi malaria parasites. We discovered for the first time that N-251 specifically inhibits the trophozoite stage of P. chabaudi, and this parasite is accordingly suggested as a model for the study of stage-specific antimalarial effects in mice.
Notes
-
The authors have no conflict of interest.
-
Author contributions
Conceptualization: Aly N, Sato A, Miyoshi SI, Chang KS, Yu HS, Kobayashi F, Kim HS
Data curation: Aly N, Sato A, Kim HS
Formal analysis: Aly N, Sato A, Kim HS
Funding acquisition: Dinh TQ, Kim HS
Investigation: Aly N, Matsumori H, Dinh TQ, Sato A
Methodology: Aly N, Matsumori H, Dinh TQ
Project administration: Kim HS
Resources: Matsumori H, Dinh TQ, Sato A, Miyoshi SI, Kobayashi F, Kim HS
Software: Matsumori H, Dinh TQ, Chang KS, Yu HS, Kobayashi F
Supervision: Kim HS
Validation: Aly N
Visualization: Aly N
Writing – original draft: Aly N
Writing – review & editing: Aly N, Miyoshi SI, Chang KS, Yu HS, Kobayashi F, Kim HS
Acknowledgments
This study was partially supported by a grant from the Program of the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID, JP22wm0125004) from the Ministry of Education, Culture, Sports, Science, and Technology in Japan (MEXT), and the Japan Agency for Medical Research and Development (AMED). We thank Taiki Osato (Okayama University) for the partial experiments and discussion.
Fig. 1Structure of N-251. The chemical structure of 6-(1,2,6,7-tetraoxaspiro [7.11] nonadec-4-yl) hexan-1-ol (N-251) [
7].
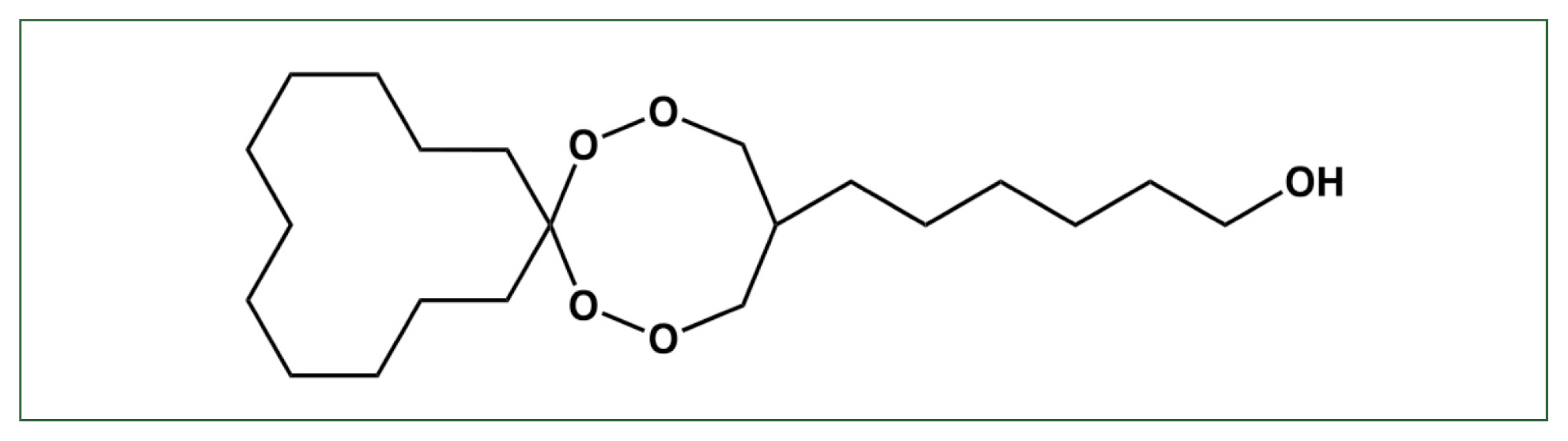
Fig. 2Scheme for analyzing the stage-specific effect of N-251. The drug was administered at a dose of 68 mg/kg every 8 h for 1 day. The control group was treated with vehicle only at the time indicated by the scheme, parasitemias, and parasite stage distributions were found. (A) Parasites have many ring stages. (B) Parasites abundant in the trophozoite stage.
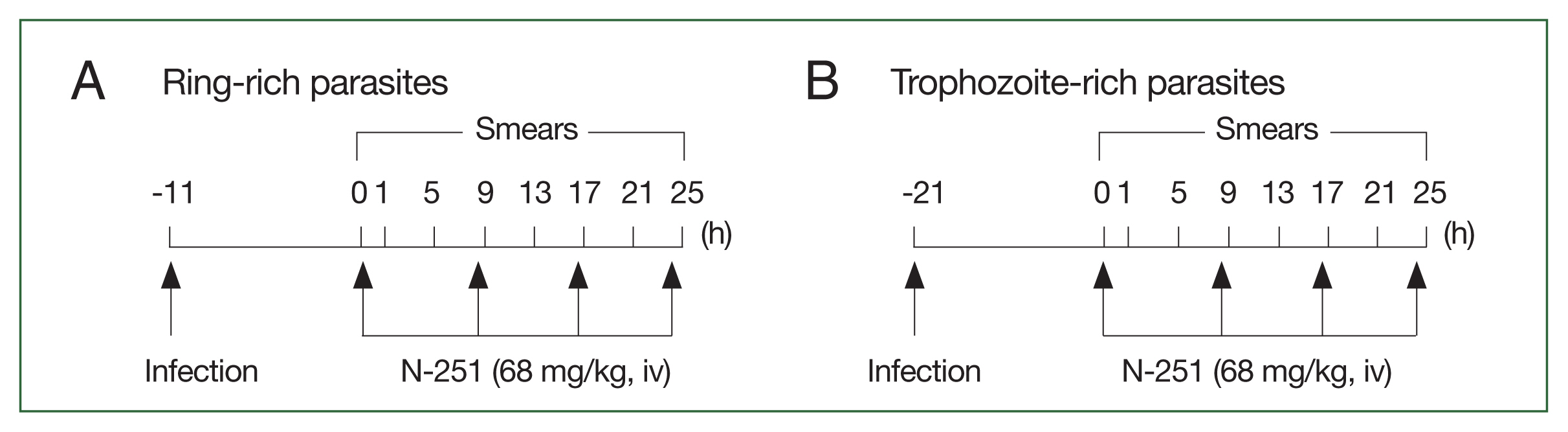
Fig. 3
P. chabaudi and P. berghei growth curves. Squares represent P. chabaudi and circles represent P. berghei. Mice were infected by iv infusion of 1×106 infected erythrocytes. A thin smear was prepared and the parasitemia was assessed under a microscope. Data were expressed as the mean with a bar showing the SD value.
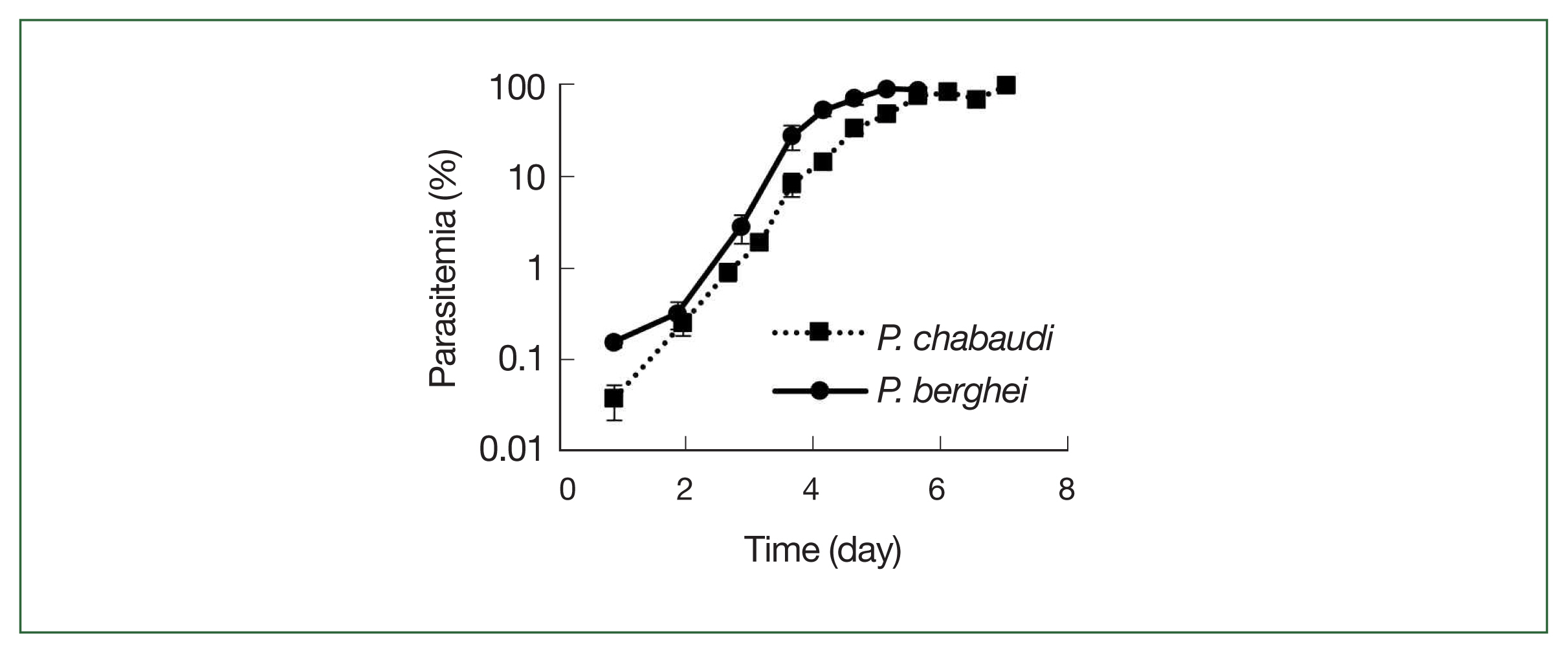
Fig. 4Effect of melatonin on asynchronized P. chabaudi and P. berghei parasites. Mice were infected by iv infusion of 3×105 infected erythrocytes and treated with melatonin (50 mg/kg/day for 4 consecutive days). Only a vehicle was used to treat the control group. Parasitemia and distributions of parasite stages were determined on day 4. (A) P. chabaudi parasites. (B) P. berghei parasites. *Parasitemia. Data were expressed as the mean with a bar revealing the SD value.
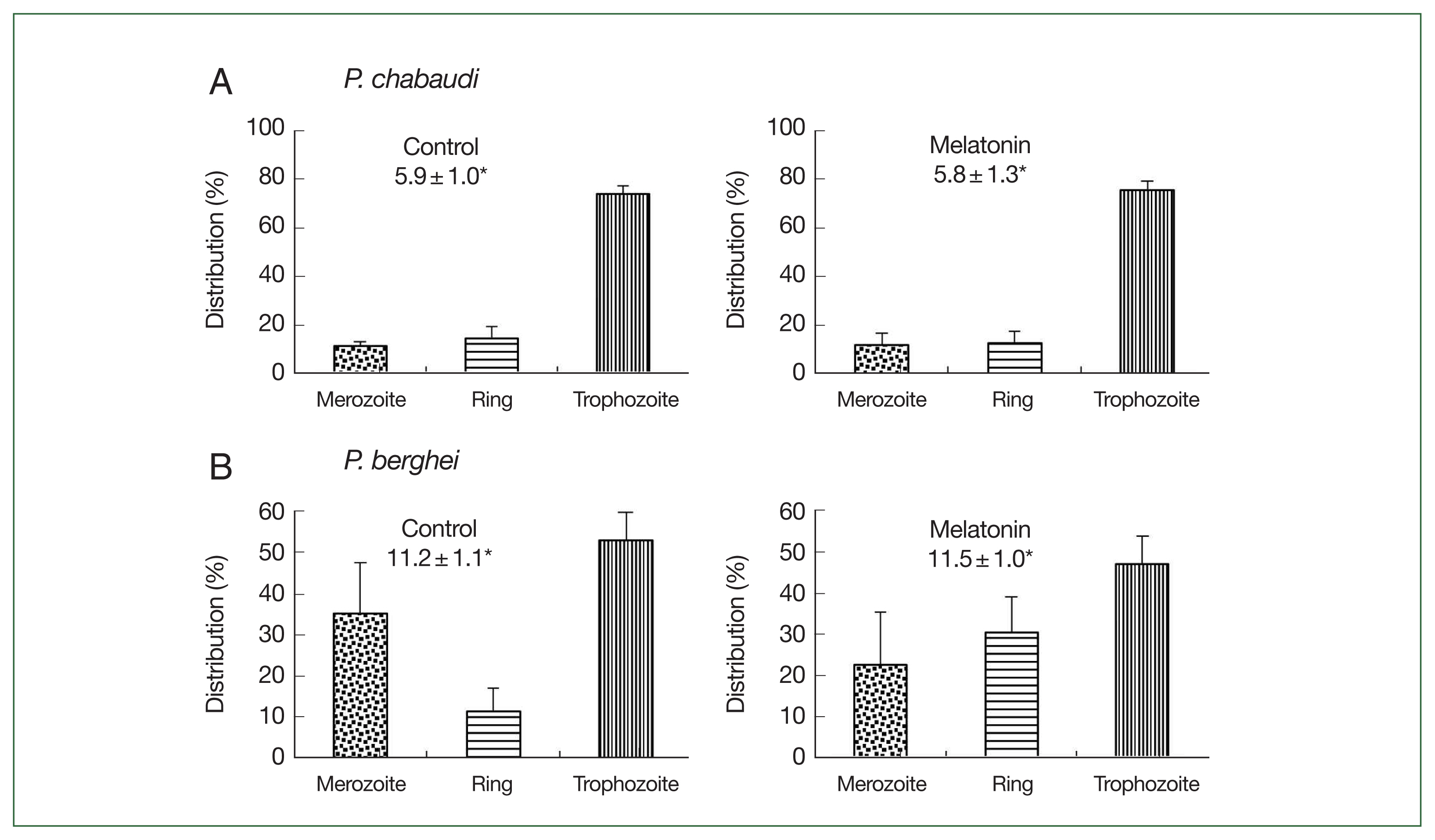
Fig. 5Stage-specific effect of N-251 on ring-stage- and trophozoite stage-rich P. chabaudi. Mice were infected by iv infusion of 1×107 infected erythrocytes. The drug was given at a dose of 68 mg/kg every 8 h for 1 day. Only a vehicle was used for the control group’s treatment. (A) Ring-stage-rich parasites. (B) Trophozoite-stage-rich parasites. For the therapy of the control group, only a vehicle was utilized. parasites with rich ring stages.
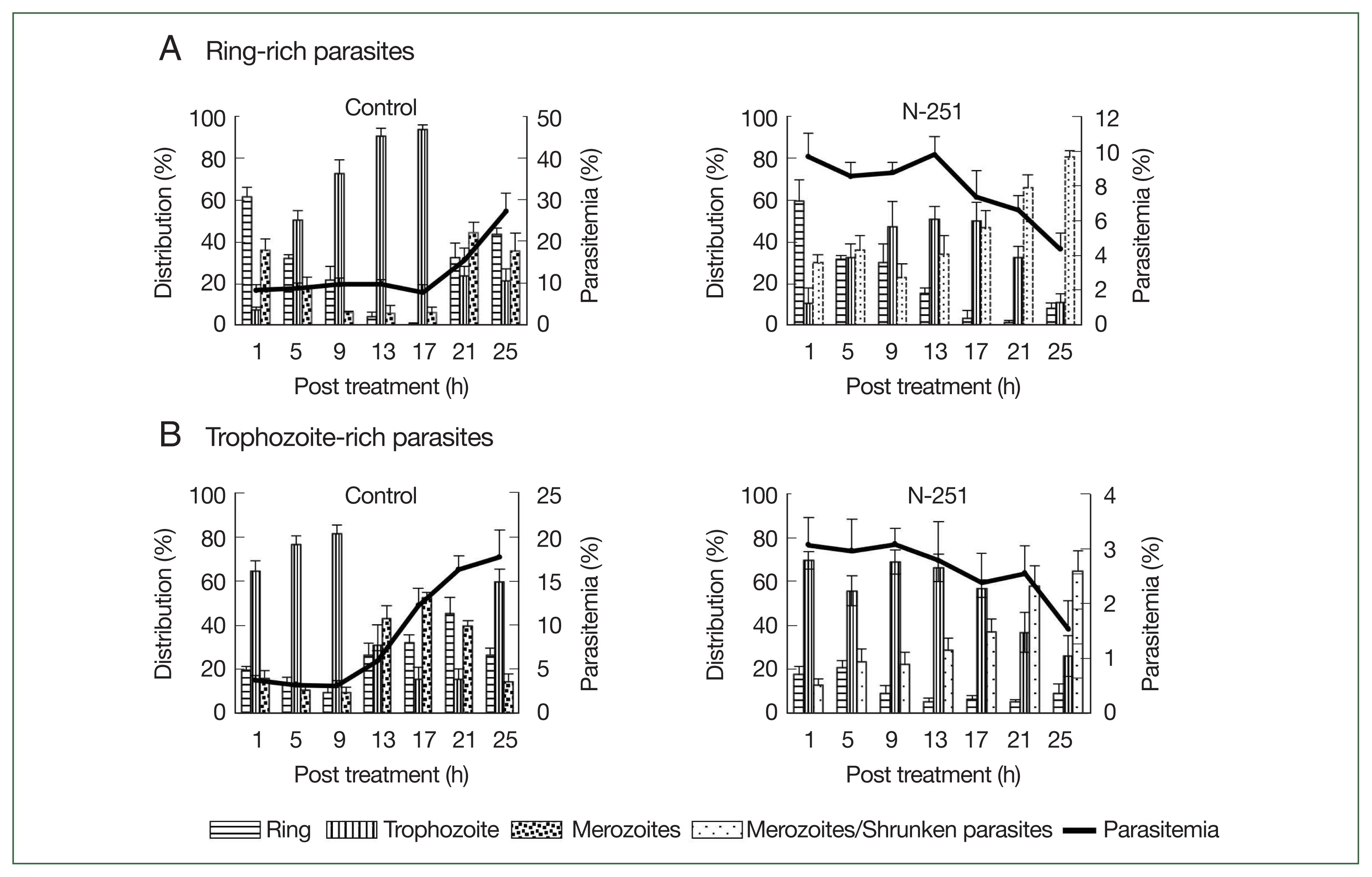
Table 1Effect of N-251 on parasitic rodent malaria. Using a 4-day suppression test, the in vivo antimalarial activity of N-251 was evaluated against asynchronized P. berghei and ring-stage- and trophozoite-stage-rich P. chabaudi
Table 1
|
N-251 (mg/kg) |
P. berghei
|
P. chabaudi
|
|
|
|
Asynchronized |
Ring-rich*
|
Trophozoite-rich*
|
|
ED50
|
22.0±2.0 |
27.0±4.0 |
22.0±3.0 |
|
|
ED90
|
45.0±1.0 |
48.0±2.0 |
47.0±2.0 |
References
- 1. Hodel EM, Kay K, Hastings IM. 2016 Incorporating stage-specific drug action into pharmacological modeling of antimalarial drug treatment. Antimicrob Agents Chemother 2016;60(5):2747-2756. https://doi.org/10.1128/AAC.01172-15
- 2. Moreno A, Badell E, Van Rooijen N, Druilhe P. Human malaria in immunocompromised mice: new in vivo model for chemotherapy studies. Antimicrob Agents Chemother 2001;45(6):1847-1853. https://doi.org/10.1128/AAC.45.6.1847-1853.2001
- 3. Fidock DA, Rosenthal PJ, Croft SL, Brun R, Nwaka S. Antimalarial drug discovery: efficacy models for compound screening. Nat Rev Drug Discov 2004;3(6):509-520. https://doi.org/10.1038/nrd1416
- 4. Wengelnik K, Vidal V, Ancelin ML, Cathiard AM, Morgat JL, et al. A class of potent antimalarials and their specific accumulation in infected erythrocytes. Science 2002;295(5558):1311-1314. https://doi.org/10.1126/science.1067236
- 5. Owolabi ATY, Reece SE, Schneider P. Daily rhythms of both host and parasite affect antimalarial drug efficacy. Evol Med Public Health 2021;9(1):208-219. https://doi.org/10.1093/emph/eoab013
- 6. Wilson DW, Langer C, Goodman CD, McFadden GI, Beeson JG. Defining the timing of action of antimalarial drugs against Plasmodium falciparum. Antimicrob Agents Chemother 2013;57(3):1455-1467. https://doi.org/10.1128/AAC.01881-12
- 7. Kim HS, Nagai Y, Ono K, Begum K, Wataya Y, et al. Synthesis and antimalarial activity of novel medium-sized 1,2,4,5-tetraoxacycloalkanes. J Med Chem 2001;44(14):2357-2361. https://doi.org/10.1021/jm010026g
- 8. Sato A, Hiramoto A, Morita M, Matsumoto M, Komich Y, et al. Antimalarial activity of endoperoxide compound 6-(1,2,6,7-tetraoxaspiro [7.11] nonadec-4-yl) hexan-1-ol. Parasitol Int 2011;60(3):270-273. https://doi.org/10.1016/j.parint.2011.04.001
- 9. Morita M, Koyama T, Sanai H, Sato A, Hiramoto A. Stage specific activity of synthetic antimalarial endoperoxides, N-89 and N-251, against Plasmodium falciparum. Parasitol Int 2015;64(1):113-117. https://doi.org/10.1016/j.parint.2014.10.007
- 10. Leelaviwat N, Mekraksakit P, Cross KM, Landis DM, McLain M, et al. Melatonin: Translation of ongoing studies into possible therapeutic applications outside sleep disorders. Clin Ther 2022;44(5):783-812. https://doi.org/10.1016/j.clinthera.2022.03.008
- 11. Hotta CT, Gazarini ML, Beraldo FH, Varotti FP, Lopes C, et al. Calcium-dependent modulation by melatonin of the circadian rhythm in malarial parasites. Nature Cell Biol 2000;2(7):466-468. https://doi.org/10.1038/35017112
- 12. Mallaupoma LRC, Dias BKM, Singh MK, Honorio RI, Nakabashi M, et al. Decoding the role of melatonin structure on Plasmodium falciparum human malaria parasites synchronization using 2-sulfenylindoles derivatives. Biomolecules 2022;12(5):638. https://doi.org/10.3390/biom12050638
- 13. Peters W. The chemotherapy of rodent malaria, XXII. The value of drug-resistant strains of P. berghei in screening for blood schizontocidal activity. Ann Trop Med Parasitol 1975;69(2):155-171.
- 14. Smith LM, Motta FC, Chopra G, Moch JK, Nerem RR, et al. An intrinsic oscillator drives the blood stage cycle of the malaria parasite Plasmodium falciparum. Science 2020;368(6492):754-759. https://doi.org/10.1126/science.aba4357
- 15. Beraldo FH, Almeida FM, da Silva AM, Garcia CR. Cyclic AMP and calcium interplay as second messengers in melatonin-dependent regulation of Plasmodium falciparum cell cycle. J Cell Biol 2005;170(4):551-557. https://doi.org/10.1083/jcb.200505117
- 16. Singh MK, Dias BKM, Garcia CRS. Role of melatonin in the synchronization of asexual forms in the parasite Plasmodium falciparum. Biomolecules 2020;10(9):1243. https://doi.org/10.3390/biom10091243
- 17. Gibbs FP, Vriend J. The half-life of melatonin elimination from rat plasma. Endocrinology 1981;109(5):1796-1798. https://doi.org/10.1210/endo-109-5-1796
- 18. Andersen LP, Gögenur I, Rosenberg J, Reiter RJ. The safety of melatonin in humans. Clin Drug Investig 2016;36(3):169-175. https://doi.org/10.1007/s40261-015-0368-5
- 19. Pereira PHS, Garcia CRS. Melatonin action in Plasmodium infection: Searching for molecules that modulate the asexual cycle as a strategy to impair the parasite cycle. J Pineal Res 2021;70(1):e12700. https://doi.org/10.1111/jpi.12700
- 20. Bagnaresi P, Alves E, Borges da Silva H, Epiphanio S, et al. Unlike the synchronous Plasmodium falciparum and P. chabaudi infection, the P. berghei and P. yoelii asynchronous infections are not affected by melatonin. Int J Gen Med 2009;2:47-55. https://doi.org/10.2147/ijgm.s3699
- 21. Shuto S, Minakawa N, Niizuma S, Kim HS, Wataya Y, et al. New neplanocin analogs 12. An alternative synthesis of (6′R)-6′-C-methylneplanocin A (RMNPA), a novel potent anti-malarial agent. J Med Chem 2002;45(3):748-751. https://doi.org/10.1021/jm010374i
- 22. Kim HS, Shibata Y, Wataya Y, Tsuchiya K, Masuyama A, et al. Synthesis and antimalarial activity of cyclic peroxides, 1,2,4,5,7-pentoxocanes and 1,2,4,5-tetroxanes. J Med Chem 1999;42(14):2604-2609. https://doi.org/10.1021/jm990014j
- 23. Tsuchiya K, Hamada Y, Masuyama A, Nojima M, McCullough KJ, et al. Synthesis, crystal structure and anti-malarial activity of novel spiro-1,2,4,5-tetraoxacycloalkanes. Tetrahedron Lett 1999;40(21):4077-4080. https://doi.org/10.1016/S0040-4039(99)00653-X
- 24. Miyaoka H, Shimomura M, Kimura H, Yamada Y, Kim HS, et al. Antimalarial activity of kalihinol A and new relative diterpenoids from the okinawan sponge, Acanthella sp. Tetrahedron 1998;54(44):13467-13474. https://doi.org/10.1016/S0040-4020(98)00818-7
- 25. Aly NS, Hiramoto A, Sanai H, Hiraoka O, Hiramoto K, et al. Proteome analysis of new antimalarial endoperoxide against Plasmodium falciparum. Parasitol Res 2007;100(5):1119-1124. https://doi.org/10.1007/s00436-007-0460-8
- 26. Morita M, Sanai H, Hiramoto A, Sato A, Hiraoka O, et al. Plasmodium falciparum endoplasmic reticulum-resident calcium binding protein is a possible target of synthetic antimalarial endoperoxides, N-89 and N-251. J Proteome Res 2012;11(12):5704-5711. https://doi.org/10.1021/pr3005315
 , Hiroaki Matsumori1, Thi Quyen Dinh1, Akira Sato1,3, Shin-Ichi Miyoshi4, Kyung-Soo Chang5, Hak Sun Yu6, Fumie Kobayashi7, Hye-Sook Kim1,*
, Hiroaki Matsumori1, Thi Quyen Dinh1, Akira Sato1,3, Shin-Ichi Miyoshi4, Kyung-Soo Chang5, Hak Sun Yu6, Fumie Kobayashi7, Hye-Sook Kim1,*