Abstract
Lophomonas blattarum is an anaerobic protozoan living in the intestine of cockroaches and house dust mites, with ultramicroscopic characteristics such as the presence of a parabasal body, axial filament, and absence of mitochondria. More than 200 cases of Lophomonas infection of the respiratory tract have been reported worldwide. However, the current diagnosis of such infection depends only on light microscopic morphological findings from respiratory secretions. In this study, we attempted to provide more robust evidence of protozoal infection in an immunocompromised patient with atypical pneumonia, positive for Lophomonas-like protozoal cell forms. A direct search of bronchoalveolar lavage fluid via polymerase chain reaction (PCR), transmission electron microscopy (TEM), and metagenomic next-generation sequencing did not prove the presence of protozoal infection. PCR results were not validated with sufficient rigor, while de novo assembly and taxonomic classification results did not confirm the presence of an unidentified pathogen. The TEM results implied that such protozoal forms in light microscopy are actually non-detached ciliated epithelial cells. After ruling out infectious causes, the patient’s final diagnosis was drug-induced pneumonitis. These findings underscore the lack of validation in the previously utilized diagnostic methods, and more evidence in the presence of L. blattarum is required to further prove its pathogenicity.
-
Key words: Lophomonas blattarum, bronchial ciliated epithelium, ultrastructure, misdiagnosis
Lophomonas blattarum is an anaerobic protozoan first identified in the 1860s in the cockroach intestine [
1]. Under light microscopy, it is about 20 to 60 μm in length, has an oval to round shape and more than 50 flagella located at its anterior extremity [
2]. Its characteristic organelles such as parabasal body, axial filament, and absence of mitochondria were revealed by transmission electron microscopy (TEM) studies [
3,
4].
L. blattarum emerged as a possible opportunistic pathogen of the human lower respiratory tract in the 1990s. More than 200 cases of
Lophomonas infection have been reported worldwide. The reported prevalence of
Lophomonas infection in patients with respiratory symptoms in China, Peru, Iran, Mexico and India was 8% to 30%, mostly in pediatric and immunocompromised patients [
5,
6].
The current diagnostic scheme for
Lophomonas infection is primarily based on the morphology of cells observed by light microscopy on a wet smear or different staining techniques in patients with a medical history that raises suspicion of such infection [
7,
8]. However, concerns about misdiagnosis have repeatedly been raised [
9–
11] due to the lack of definitive diagnostic methods. In this paper, we report a patient suspected of having pulmonary lophomoniasis. Via electron microscopy, PCR, and metagenomic next-generation sequencing (NGS), we suggest that the previously implemented approach involving light microscopy and polymerase chain reaction (PCR) cannot distinguish true infection from respiratory epithelial cells with an unconventional morphology.
A leftover patient sample from diagnostic bronchoalveolar lavage fluid (BALF) collected by flexible bronchoscopy was used in this study. Normal blood and BALF samples were also used as controls. The patient was a 48-year-old Korean female who visited an outpatient clinic of the Seoul National University Bundang Hospital Lung Center with the chief complaints of cough and sputum discharge, which had started a month previously. The patient had suffered from ulcerative colitis for 5 years, had been treated with sulfasalazine and corticosteroid therapy for 4 years, and had initiated infliximab therapy 10 months prior to the visit with steroid tapering. She did not report any history of respiratory disease. Multiple patchy nodular consolidations were observed in chest X-ray and computed tomography (CT). Atypical pneumonia was suspected, but the initial sputum and acid-fast bacilli culture were negative. She did not have a fever or other signs of infection. Laboratory results showed a normal complete blood count, with slightly increased aspartate aminotransferase and alanine aminotransferase levels of 52 and 87 IU/L, respectively.
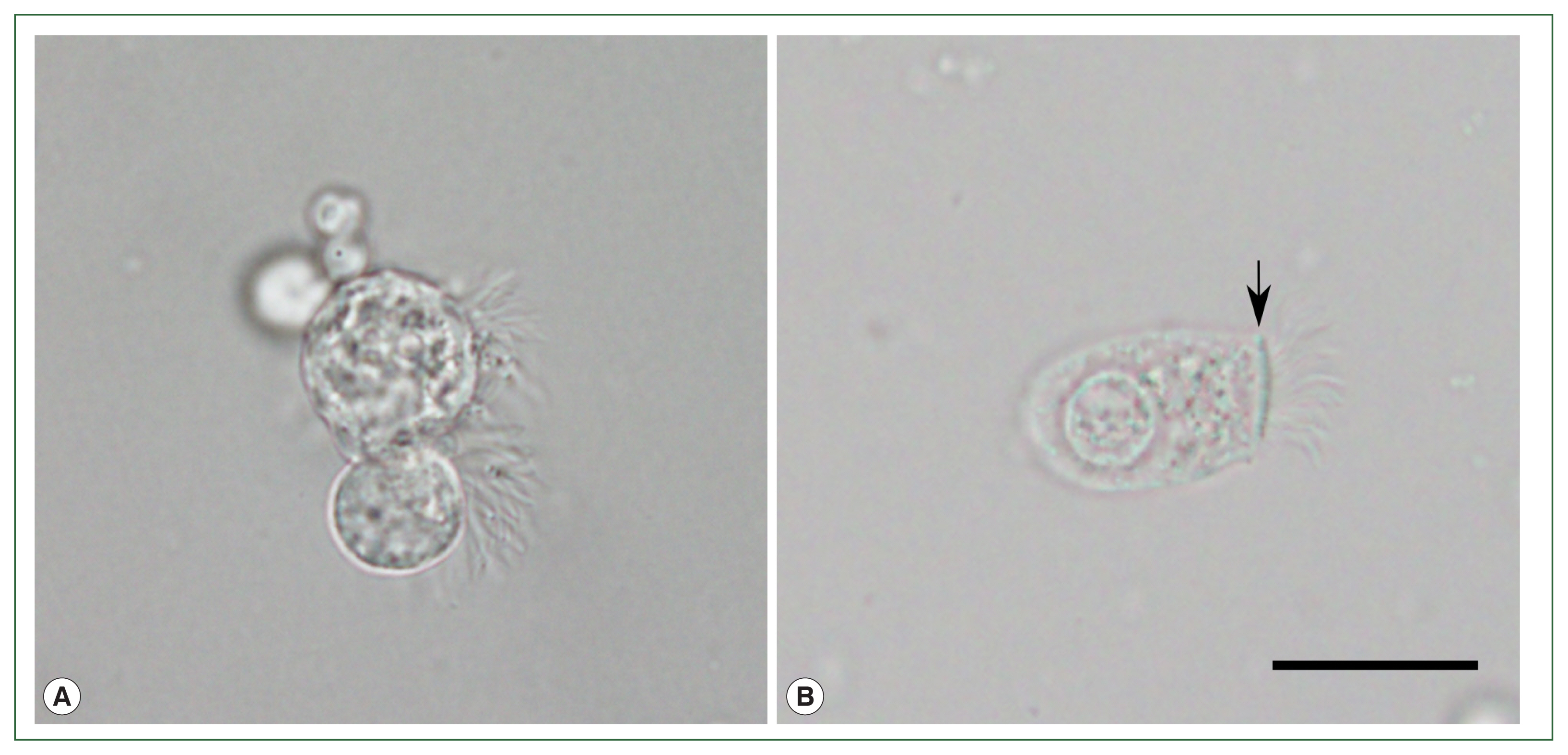
The initial 14-day treatment of cefpodoxime failed to relieve the symptoms. Diagnostic flexible bronchoscopy was performed, and multiple protozoon-like cellular organisms were observed in the BALF under a light microscope (BX53; Olympus Co., Tokyo, Japan;
Fig. 1A). The cells were approximately 20 to 40 μm in size, and had a round to pear-shaped body and flagella inserted in various directions without the presence of a terminal bar. Multiple vacuole-like organelles hindered detailed observation of the cellular structures. The cells showed unsynchronized, jerking ciliary movement under a wet mounting slide. Such findings coincided with the morphological features of
L. blattarum, while being clearly distinct from ciliated epithelial cells with a normal morphology (
Fig. 1B). The patient did not report significant contact with cockroaches or any recent changes in her living or working environment. Based on these observations, 14-day metronidazole therapy targeting protozoal infection was initiated, and the patient reported a decrease in the amount of sputum discharge. However, other symptoms persisted and chest CT showed no improvement. Drug-induced pneumonitis caused by recently initiated infliximab was suspected, so the treatment was stopped. The findings on chest X-ray and CT slowly improved over 1 month, and respiratory symptoms completely subsided after 2 months. The patient was finally diagnosed with infliximab-induced pneumonitis.
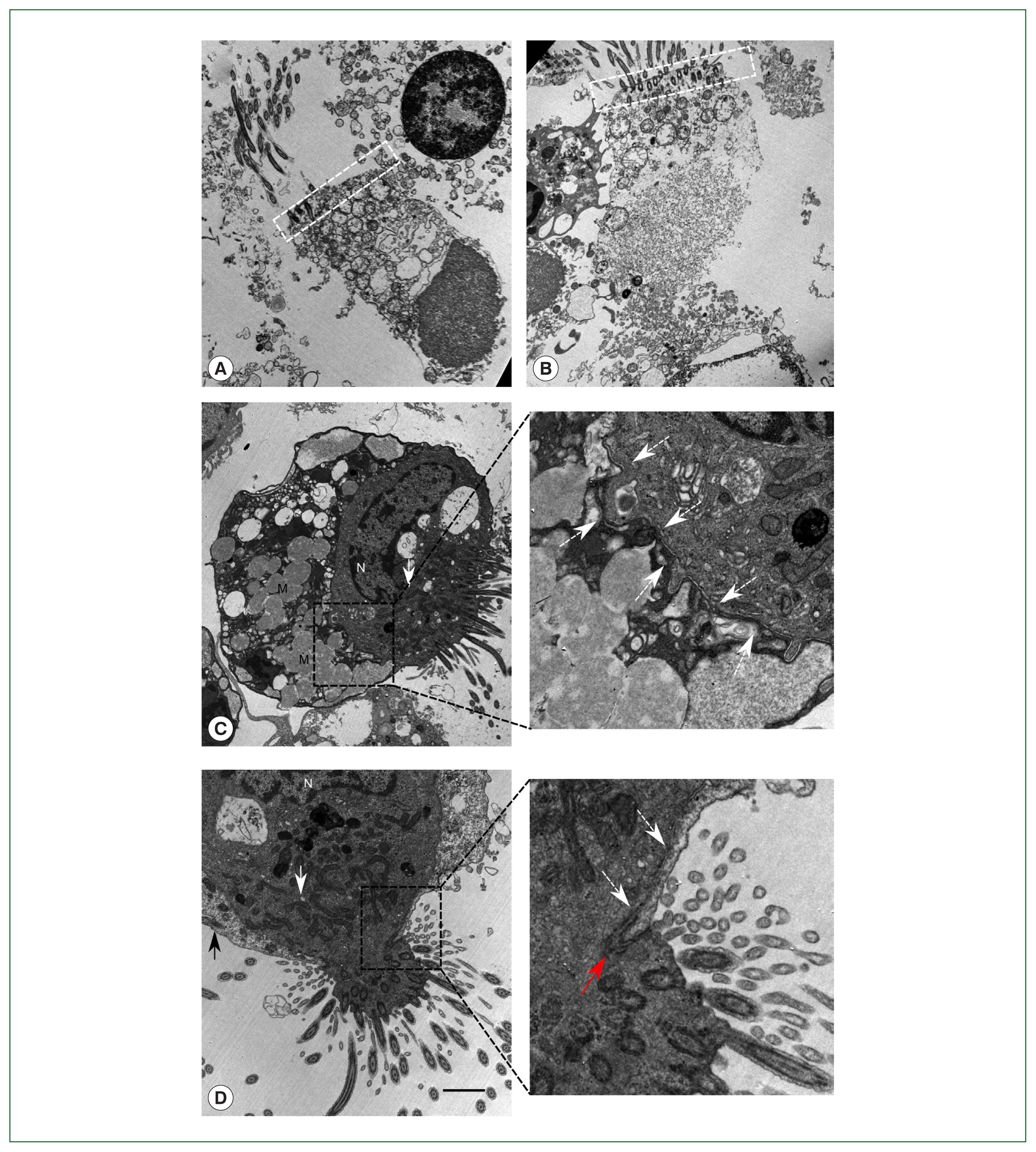
To confirm the presence of
Lophomonas, methods other than light microscopy were attempted. TEM was performed to search for ultrastructural features representative of
Lophomonas. Ten milliliters of the patient’s BALF were centrifuged at 300×g for 15 min. The sediment was fixed by adding 3% glutaraldehyde and 1% osmium tetroxide. The sample was dehydrated with ethanol and acetone, embedded with epoxy resin 618, sliced with an ultramicrotome, stained with 3% uranyl acetate and Reynolds’s lead citrate, and observed under a transmission electron microscope (JEM-1400; Japan Electron Optics Laboratory Co., Tokyo, Japan). We observed many ciliated bronchial epithelial cells from the patient’s BALF samples: normal ciliated epithelial cells about 20 μm in size, typical structural arrangement with a terminal bar, insertion of multiple cilia, mitochondria concentrated in the apical region, and nucleus in the basal region (
Fig. 2A). Some epithelial cells showed a detached nucleus, which is an example of ciliocytophthoria (
Fig. 2B). Atypical ciliated cells leading to a suspicion of
Lophomonas infection were also observed. However, the cells clearly did not show the characteristics of
L. blattarum. One of the atypical
Lophomonas-like cells had multiple mucinous vacuoles resembling goblet cells (
Fig. 2C). Meanwhile, another cell showed a tight junction structure, providing clear evidence that the
Lophomonas-like atypical cells consisted of 2 or more conjoined cells (
Fig. 2D). A magnified image showed a clear boundary of the cell membranes, which never crossed each other. These ciliated epithelial cells had lost the regular terminal bar structure and had a non-linear ciliary insertion angle mimicking the ciliary tuft of
Lophomonas. The TEM results showed that the
Lophomonas-like protozoal cells were actually non-detached fragments of the ciliary epithelial lining.
A recently reported PCR method and primers from Iran were utilized [
6,
15,
16]. The authors claimed that the primers were specifically designed to detect
L. blattarum genome. A total of 500 μl of the patient’s BALF was used to extract DNA with an EMAG automated DNA extraction kit (bioMérieux, Marcy l’Etoile, France). Recombinant
Taq polymerase, dNTP mixture, and reaction buffers were mixed and used in accordance with the manufacturer’s instructions (Takara Bio, Shiga, Japan). PCR forward and reverse primer sequences were 5′-GAGAA GGCGC CTGAG AGAT-3′ and reverse (R) 5′-ATGGG AGCAA ACTCG CAGA-3′, respectively, with amplification conditions as described previously [
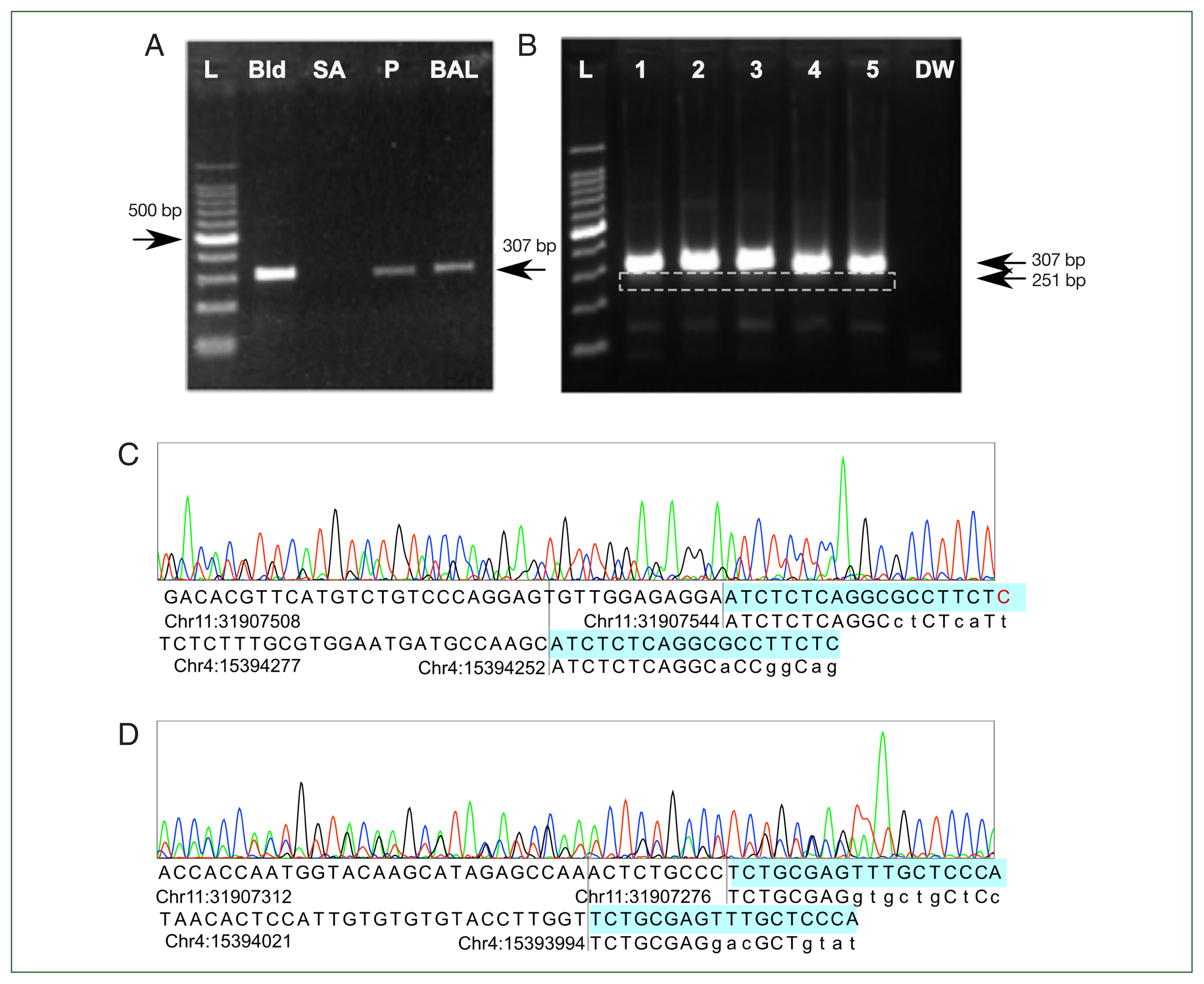
15]. PCR on both the patient’s BALF and negative control DNA from human blood produced a 300-bp-sized band. PCR with DNA extracted from a
Staphylococcus aureus colony was negative (
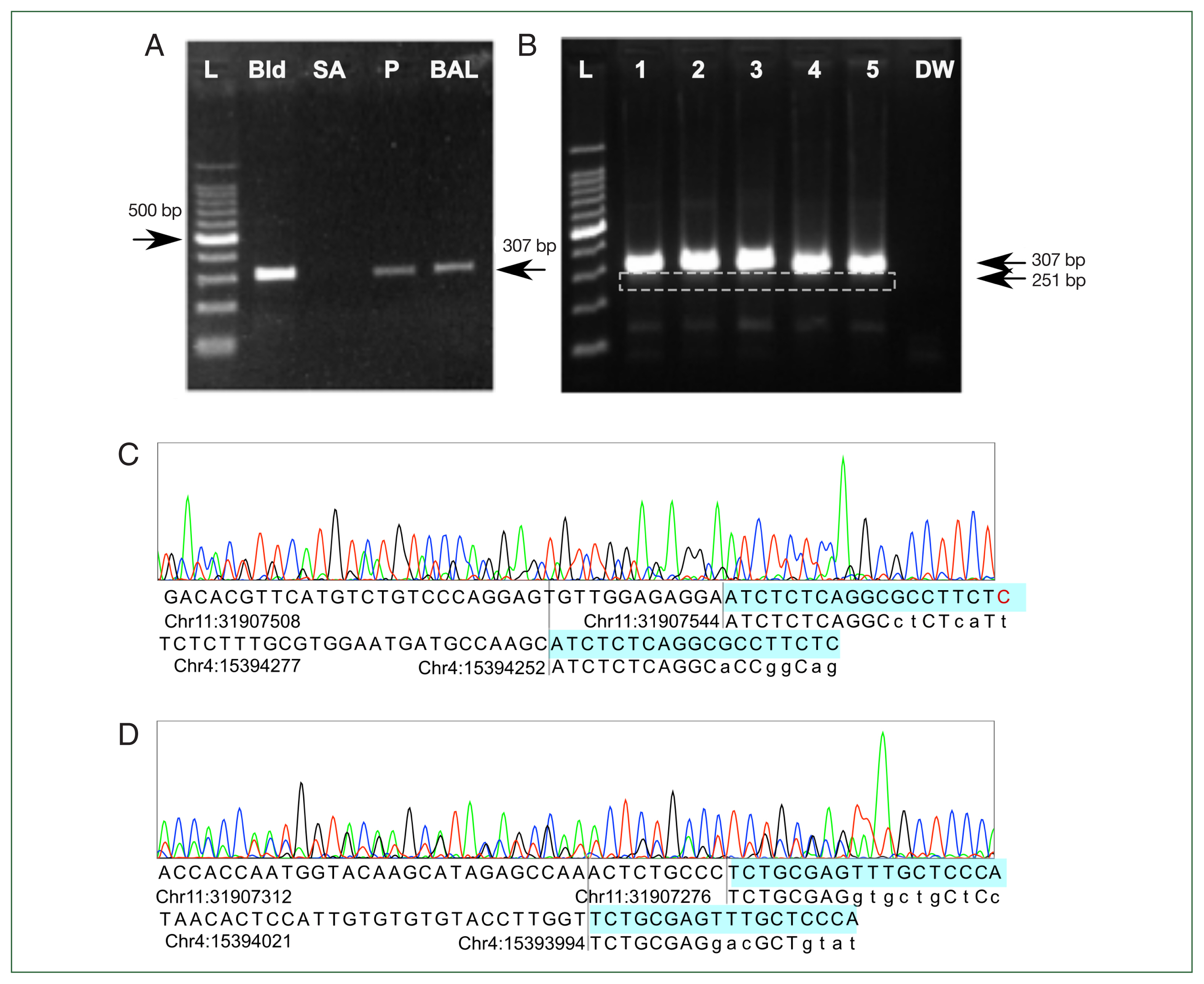
Fig. 3A). Another validation set with DNA from leftover BALF from 3 patients without flagellated microorganism in microscopic inspection and 2 samples of normal human blood showed the same amplification pattern (
Fig. 3B). Sanger sequencing of the sample from lane 5 showed multiple chromatogram peaks throughout all positions. Detailed peak examination yielded 2 different products: a 269-bp product amplifying chr11: 31907276– 31907544 and a 213-bp product in chr4: 15394040–15394252, consistent with 307-bp and 251-bp products considering the lengths of the forward and reverse primers (
Fig. 3C). Adding the lengths of the forward and reverse primers explained the 307- and 251-bp products in the gel electrophoresis results. The predicted annealing site in the hg19 genome matched more than 8 bases in the 3’ end of the forward and reverse primers, but the exact match was only about 70%. This might have prevented a positive result from being obtained in the in silico PCR prediction. In addition, NGS-based metagenomic analysis using de novo assembly and taxonomic classification was attempted [
17], but failed to reveal the presence of
L. blattarum (data not shown).
The current diagnostic scheme of L. blattarum infection depends solely on light microscopy and patient history, but its validity has long been debated. In this study, multiple methods other than light microscopy were utilized in order to look for robust evidence of Lophomonas infection.
PCR is the most applicable method for detecting
L. blattarum. A PCR primer sequence pair and molecular diagnostic scheme were reported in 2019 based on a public SSU-rRNA sequence, but raw sequence data and a peak diagram for the amplified PCR results were not provided [
6,
15,
16]. In the studies, neither positive
Lophomonas-infected control from validated patients nor pure
Lophomonas DNA from the cockroach intestine was tested. Moreover, the validity of the reference sequence is questionable. A search in NIH GenBank showed the 328-bp sequence used to generate the primers. This sequence was not published and was submitted from Thailand in May 2012. The origin and validity of the sequence reference require further validation. Since the primers achieved amplification for the normal human control sample in our study, the reliability of previous studies using the same primers is also questionable.
To design different primer sets, in vitro pure culture and the genomic sequence of the protozoa are required. The method of culturing
L. blattarum was reported in the 1950s but, in the past 50 years, no study has reported the success of pure culture from patient-derived specimens or even cockroach intestinal specimens [
18]. Metagenomic NGS also failed.
Nonetheless, observation of the characteristic parabasal body, axial filament, and absence of mitochondria from a patient-derived specimen could provide unassailable evidence [
3,
4]. To our knowledge, this is the first attempt to observe the ultrastructure of
L. blattarum-like cells by TEM from a fresh patient sample. The results showed that the strange flagellated protozoon-like cells were most likely 2 or more conjoined cells of human bronchial lining, mimicking the protozoal organelle of
Lophomonas. This is corroborated by a study from China that utilized scanning electron microscopy. The authors concluded that the protozoa-like cells were actually ciliated epithelial cells [
19].
Infliximab-induced interstitial lung disease is a rare condition that is difficult to diagnose. Infliximab is typically used with another immune-modulating drug, many of which have pulmonary side effects [
12]. To date, only one case of interstitial lung disease in a regimen involving infliximab alone has been reported [
13]. Noninfectious pulmonary disease usually occurs before 6 months of infliximab therapy. Diagnosis is made empirically based on previous medical history, time of disease onset after infliximab initiation, and cessation of symptoms after withdrawal of the drug; most importantly, other causes should be ruled out first [
14]. In the current case, we could rule out common causes of infection via patient history, drug response, radiological examination, and microbiological examination results. Morphological examinations of BALF led us to suspect
L. blattarum infection, but we could not prove the presence of the protozoa by any available means. The patient achieved complete recovery after the withdrawal of infliximab, and thus a final diagnosis of infliximab-induced pneumonitis was made.
In conclusion, awareness of the possibility of infection with L. blattarum, if confirmed to be a true human pathogen, would become an important part in the management of immunocompromised patients suffering from lower respiratory tract infection. However, evidence of L. blattarum infection based on morphological findings reported to date is controversial. Our results show the possibility of overdiagnosis in the field and highlight the need for robust evidence based on techniques such as pure culture, NGS, and TEM to prove the infectious potential of this protozoal pathogen in the human respiratory tract.
Notes
-
The authors declare no conflicts of interest related to this study.
-
Author contributions
Conceptualization: Lee M, Hwang SM, Park JH, Park JS
Data curation: Lee M, Park JH, Park JS
Formal analysis: Hwang SM, Park JH
Funding acquisition: Park JS
Investigation: Lee M, Hwang SM, Park JH, Park JS
Methodology: Lee M, Hwang SM, Park JH, Park JS
Project administration: Park JS
Resources: Hwang SM, Park JS
Software: Park JH
Supervision: Hwang SM, Park JS, Park JH, Park JS
Validation: Lee M, Park JH, Park JS
Visualization: Lee M, Park JH
Writing – original draft: Lee M, Park JS
Writing – review & editing: Lee M, Park JS
Acknowledgment
The authors are grateful for the technical assistance provided by the technicians of the hematology unit in the Department of Laboratory Medicine, Seoul National University Bundang Hospital.
Fig. 1Light microscopy observation of wet mounted bronchoalveolar lavage fluid of the patient. (A) Multiple ciliated cells with morphological features similar to previously reported Lophomonas blattarum. (B) Normal ciliated epithelial cell with columnar shape, apical nucleus, terminal bar structure (arrow), and unidirectional insertion of cilia, from another patient’s bronchoalveolar lavage fluid sample. Scale bar=20 μm.
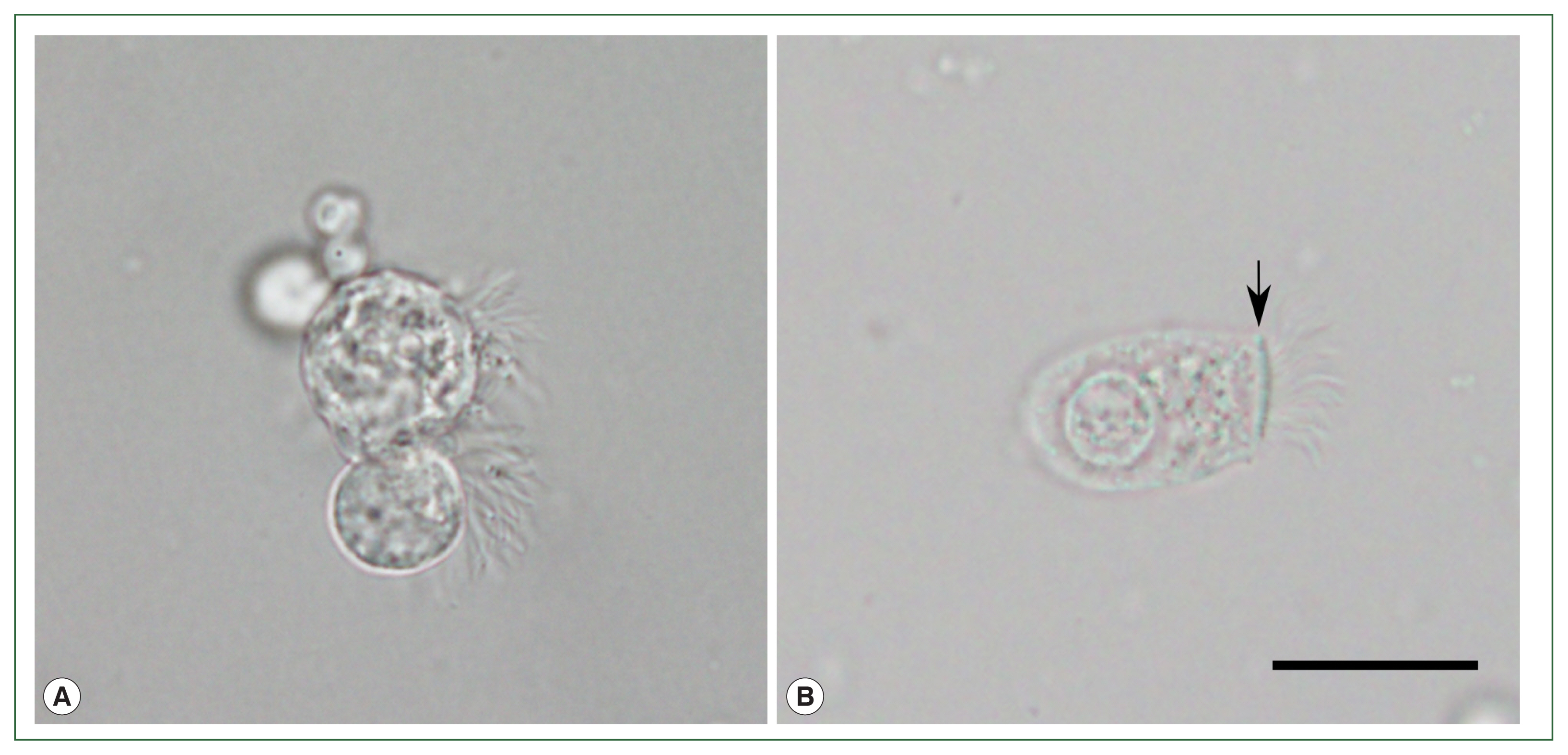
Fig. 2Findings of the patient’s bronchoalveolar lavage fluid by transmission electron microscopy. (A) A dead normal bronchial ciliated epithelial cell with the nucleus in an apical position, regular unidirectional insertion of cilia, and typical terminal bar structure (white dashed box) with nearby mitochondria. (B) Dead ciliated epithelial cell with typical morphology of ciliocytopthoria. (C) Ciliated epithelial cell tightly bound to another cell with multiple mucinous vacuoles in the cytoplasm, which resembles a goblet cell in bronchial epithelium. Cellular border is shown in the magnified region (white dashed arrow). (D) Ciliated epithelial cell bound to another ciliated cell. Both cells had lost their typical morphological features. Cellular border (white dashed arrow) and tight junction (red arrow) are shown in the magnified region. N, nucleus; M, mucinous vacuoles. White arrow indicates mitochondria gathered around the ciliary insertion site. Black arrow shows cilia in a tightly bound cell. White dashed arrow demonstrates cell membrane border. Red arrow indicates tight junction. Scale bar=2 μm.
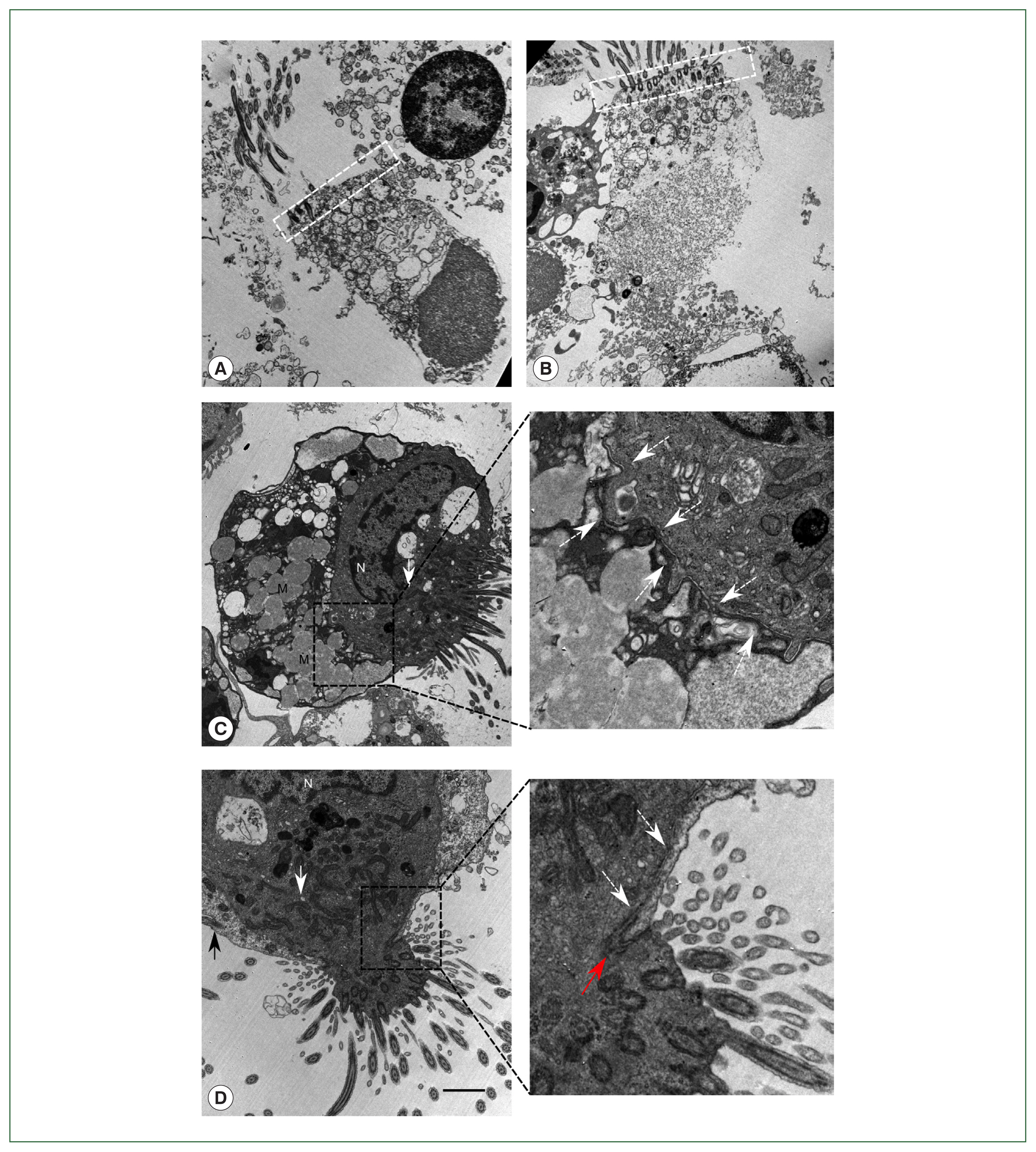
Fig. 3Polymerase chain reaction (PCR) and Sanger sequencing results. (A) Initial gel electrophoresis results show that the previously reported Lophomonas-specific primer pair reacted to all human DNA controls and produced a 307-bp product. Negative result to Staphylococcus aureus colony DNA showed that nonspecific amplification had not occurred. (B) The results are reproducible with more human DNA. Lanes 1, 2, and 3 contained DNA extracted from bronchoalveolar lavage fluid of 3 patients without lophomoniasis. Lanes 4 and 5 contained normal DNA extracted from blood. A 251-bp product (white dashed box) appeared in addition to the 307-bp product. DW was a negative control with distilled water. (C, D) Sanger sequencing results showed 2 different amplified sequences. The 307-bp product aligned to chr11: 31907276–31907544 and the 251-bp product to chr4: 15394040–15394252 in hg19. Forward and reverse primers did not fully match the genome, but at least 11 base pairs matched the genomic sequence, including >7 consecutive matches at the 3’ end. Base of primers (highlighted) matching with the human genome are capitalized. L, Ladder; Bld, control DNA extracted from healthy blood donors. SA, PCR result for DNA extracted from a Staphylococcus aureus colony. P, DNA extracted from bronchoalveolar lavage fluid from patient suspected of having Lophomonas infection. BAL, DNA extracted from Lophomonas-negative bronchoalveolar lavage fluid from another patient. DW, distilled water.
References
- 1. BüTschli O. Memoirs: researches on the flagellate infusoria and allied organisms. J Cell Sci 1879;s2-19(73):63-103. https://doi.org/10.1242/jcs.s2-19.73.63
- 2. Kudo R. Observations on Lophomonas blattarum, a flagellate inhabiting the colon of the cockroach. Blatta Orientalis Arch Protistenk 1926;53:191-214.
- 3. Beams HW, Tahmisian TN, Anderson E, Wright B. Studies on the fine structure of Lophomonas blattarum with special reference to the so-called parabasal apparatus. J Ultrastruct Res 1961;5(2):166-183. https://doi.org/10.1016/S0022-5320(61)90012-0
- 4. Beams HW, Sekhon SS. Further studies on the fine structure of Lophomonas blattarum with special reference to the so-called calyx, axial filament, and parabasal body. J Ultrastruct Res 1969;26(3):296-315. https://doi.org/10.1016/S0022-5320(69)80009-2
- 5. Ding Q, Shen K. Pulmonary Infection with Lophomonas blattarum. Indian J Pediatr 2021;88(1):23-27. https://doi.org/10.1007/s12098-020-03311-1
- 6. Fakhar M, Nakhaei M, Sharifpour A, Safanavaei S, Abedi S, Tabaripour R, Aliyali M, Modanloo M, Saberi R, Kalani H, Banimostafavi ES. Morphological and molecular identification of emerged Lophomonas blattarum infection in Mazandaran Province, northern Iran: First Registry-Based Study. Acta Parasitol 2021;66(4):1510-1516. https://doi.org/10.1007/s11686-021-00422-3
- 7. Martínez-Girón R, Ribas A, Astudillo-González A. Flagellated protozoa in cockroaches and sputum: the unhygienic connection? Allergy Asthma Proc 2007;28(5):608-609. https://doi.org/10.2500/aap2007.28.3042
- 8. Alam-Eldin YH, Abdulaziz AM. Identification criteria of the rare multi-flagellate Lophomonas blattarum: comparison of different staining techniques. Parasitol Res 2015;114(9):3309-3314. https://doi.org/10.1007/s00436-015-4554-4
- 9. Martínez-Girón R, Woerden H, Doganci L. Lophomonas misidentification in bronchoalveolar lavages. Intern Med 2011;50(21):2721. author reply 2723. https://doi.org/10.2169/internalmedicine.50.5878
- 10. Martínez-Girón R, Van Woerden HC. The burden of Lophomonas blattarum under the light microscope. J Thorac Dis 2014;6(9):E191-192. https://doi.org/10.3978/j.issn.2072-1439.2014.07.31
- 11. Gelardi M, Ciprandi G. Ciliocytophthoria of nasal epithelial cells after viral infection: a sign of suffering cell. Acta Biomed 2019;90(2-S):7-9. https://doi.org/10.23750/abm.v90i2-S.8103
- 12. Storch I, Sachar D, Katz S. Pulmonary manifestations of inflammatory bowel disease. Inflamm Bowel Dis 2003;9(2):104-115. https://doi.org/10.1097/00054725-200303000-00004
- 13. Sen S, Peltz C, Jordan K, Boes TJ. Infliximab-induced nonspecific interstitial pneumonia. Am J Med Sci 2012;344(1):75-78. https://doi.org/10.1097/MAJ.0b013e31824c07e8
- 14. Karampitsakos T, Papaioannou O, Sampsonas F, Tzouvelekis A. Infliximab-induced interstitial lung disease. BMJ Case Rep 2021;14(10):e245726. https://doi.org/10.1136/bcr-2021-245726
- 15. Fakhar M, Nakhaei M, Sharifpour A, Kalani H, Banimostafavi ES, et al. First molecular diagnosis of lophomoniasis: the end of a controversial story. Acta Parasitol 2019;64(2):390-393. https://doi.org/10.2478/s11686-019-00084-2
- 16. Nakhaei M, Fakhar M, Sharifpour A, Banimostafavi ES, Zakariaei Z, et al. First Co-morbidity of Lophomonas blattarum and COVID-19 infections: confirmed using molecular approach. Acta Parasitol 2022;67(1):535-538. https://doi.org/10.1007/s11686-021-00468-3
- 17. Lapidus AL, Korobeynikov AI. Metagenomic data assembly – the way of decoding unknown microorganisms. Front Microbiol 2021;12:613791. https://doi.org/10.3389/fmicb.2021.613791
- 18. Kirby H. Materials and Methods in the Study of Protozoa. University of California Press; California, USA. 1950, pp 23-24.
- 19. Meng SS, Dai ZF, Wang HC, Li YX, Wei DD, et al. Authenticity of pulmonary Lophomonas blattarum infection: a case report. World J Clin Cases 2019;7(1):95-101. https://doi.org/10.12998/wjcc.v7.i1.95