Abstract
Extra-gastrointestinal anisakidosis is rare. We herein report an Anisakis pegreffii infection in a patient with hepatic anisakidosis diagnosed based on its molecular identification. A 71-year-old male patient had a hepatic tumor presenting as a low-density area of 20 mm in diameter in segment 6 of the liver on abdominal ultrasonography, computed tomography, and magnetic resonance imaging. The surgically resected pathological specimen revealed a necrotizing eosinophilic granuloma containing nematode larvae, possibly an Anisakis larva. Molecular and phylogenetic analysis demonstrated Anisakis larvae belonging to A. pegreffii. The present results will help identify and characterize unknown Anisakis species in histological sections.
-
Key words: Anisakis pegreffii, hepatic anisakidosis, granuloma
Introduction
Raw or undercooked marine fish consumption may cause infection with several helminths. Anisakidosis is a foodborne disease caused by the accidental ingestion of larval nematode (commonly
Anisakis spp. or
Pseudoterranova spp.) in raw marine fishes [
1–
3]. Anisakidosis incidence continues to increase globally due to the growing popularity of consuming raw or undercooked fish [
4,
5]. Annually, over 2,000 cases of anisakidosis are assumed to occur in most regions of Japan [
3]. Approximately 95% of larvae parasitize the gastric, small intestinal, and colon mucosae, when
Anisakis larvae orally infect the digestive tract lumen. However, they occasionally perforate the intestinal wall and resulting in ectopic anisakidosis cases. By the mid-1990s, 769 cases of anisakidosis caused by
Pseudoterrranova decipiens (this species is widely known in Japan) were reported in the northern part of Japan [
3]. The ectopic form of anisakidosis is much less common than the gastric and intestinal forms, but ectopic anisakidosis caused by
Pseudoterranova spp. has been reported in humans [
6,
7]. A biopsy sample obtained under endoscopy from patients with acute or subacute abdominal symptoms is used to generally diagnose
Pseudoterranova infection. However, a previous study reported that the 4th-stage larva of
P. azarasi was expectrated by strong coughing after an asymptomatic course [
6]. To date, 3 sibling species of
Anisakis simplex larvae have been reported:
A. simplex sensu stricto (s.s.),
A. pegreffii, and
A. simplex C [
8]. The most common species in Japan is
A. simplex (s.s.) followed by
A. pegreffii, and
A. simplex (s.s.) caused 99% of all human anisakidosis [
3,
9]. The total number of anisakidosis cases reported each year is 70 in the United States of America and 500 in Europe [
1]. Herein, we report a 71-year-old male who presented with liver cancer and was diagnosed with extra-gastrointestinal anisakidosis caused by
Anisakis pegreffii.
Case Records
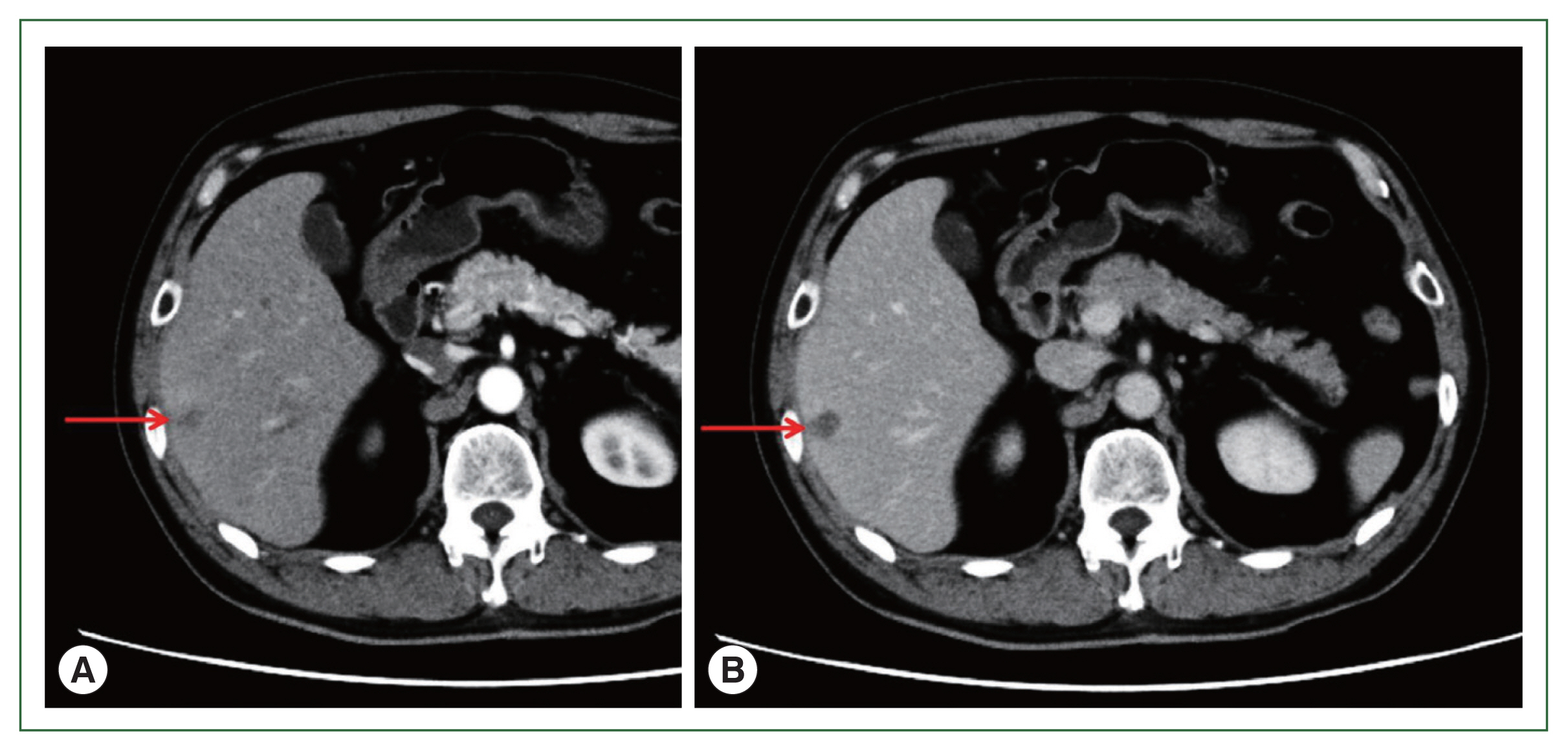
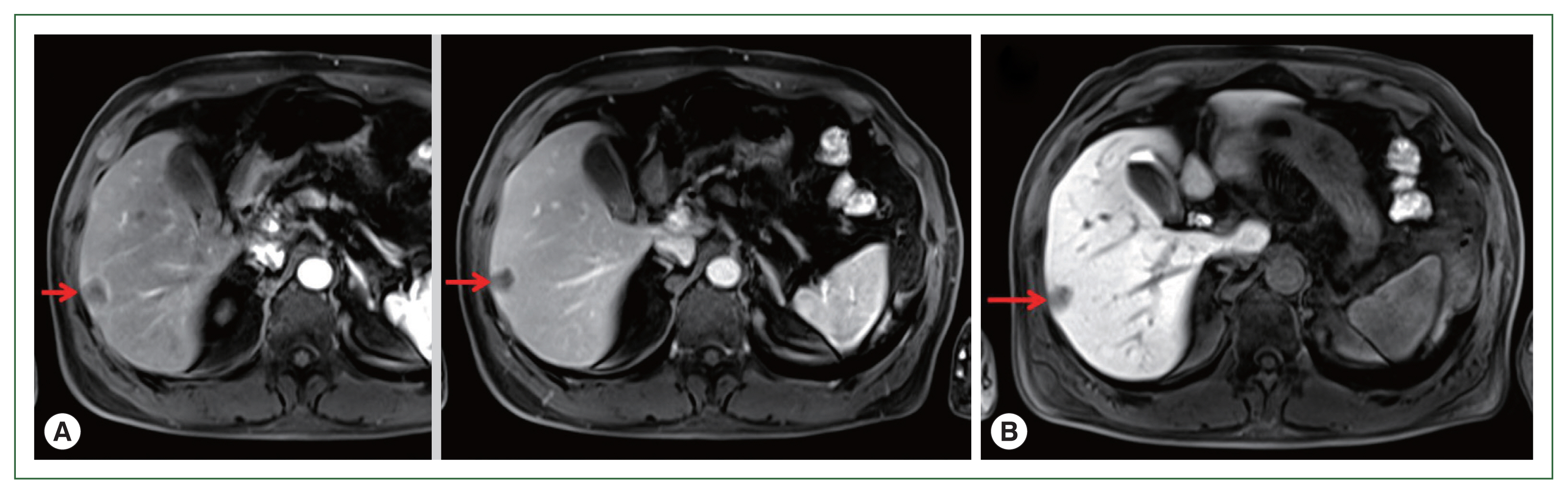
A 71-year-old male patient was admitted to Kyoto Prefectural University of Medicine Hospital with a liver tumor impression. He had a previous history of hypertension, gout, and prostatomegaly. He underwent appendectomy at 30 years of age. He had a family history of lung cancer, which affected his mother. Abdominal ultrasonography performed in health check-up revealed a low-density area of 20 mm in diameter in segment 6 of the liver. He was referred to our hospital for a more detailed lesion examination after 1 month. Difficulties were associated in reaching a definitive diagnosis although computed tomography (CT)-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (EOB-MRI) images were investigated (
Figs. 1,
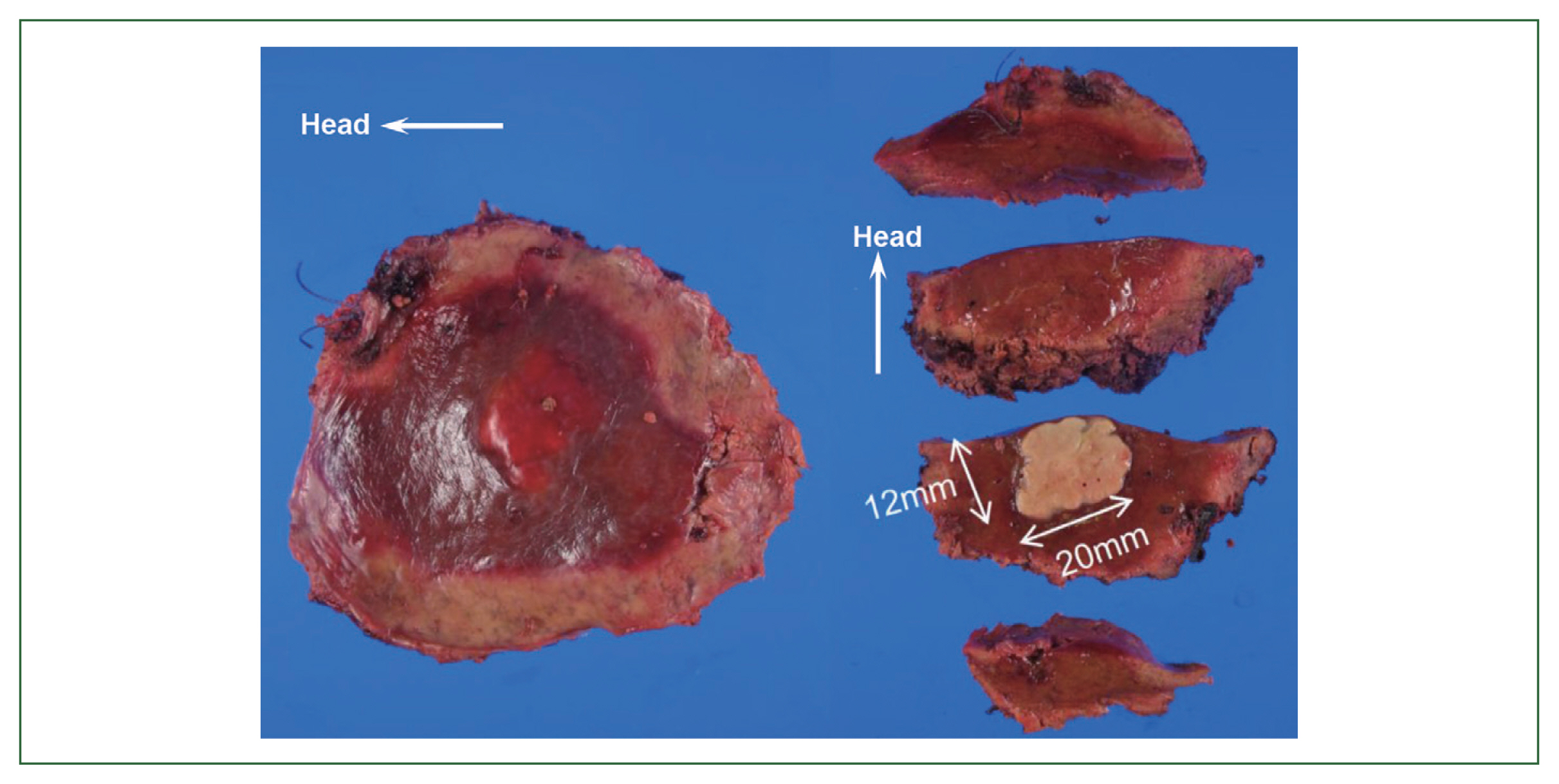
2). Therefore, a liver biopsy was performed and tissue sections were examined. Preoperative diagnosis includes a liver tumor (S6), and a differential diagnosis from hepatocellular carcinoma, metastatic liver cell carcinoma, cholangiocarcinoma in the liver, and an inflammatory pseudotumor was required. Borderline-limited necrotizing granuloma comprising coagulation necrosis containing eosinophilic ghost cells and liquefactive necrosis with a desquamated cytoplasm was revealed in a pathological examination of a tissue section from a surgical sample obtained under laparoscopy. Necrotizing granuloma of the S6 lesion in the center of the tissue section was partially resected, revealing avascular necrosis in the cavity, and contained a necrotic larval nematode with a thick pellicle and amorphous content, indicating an anisakis infection (
Figs. 3,
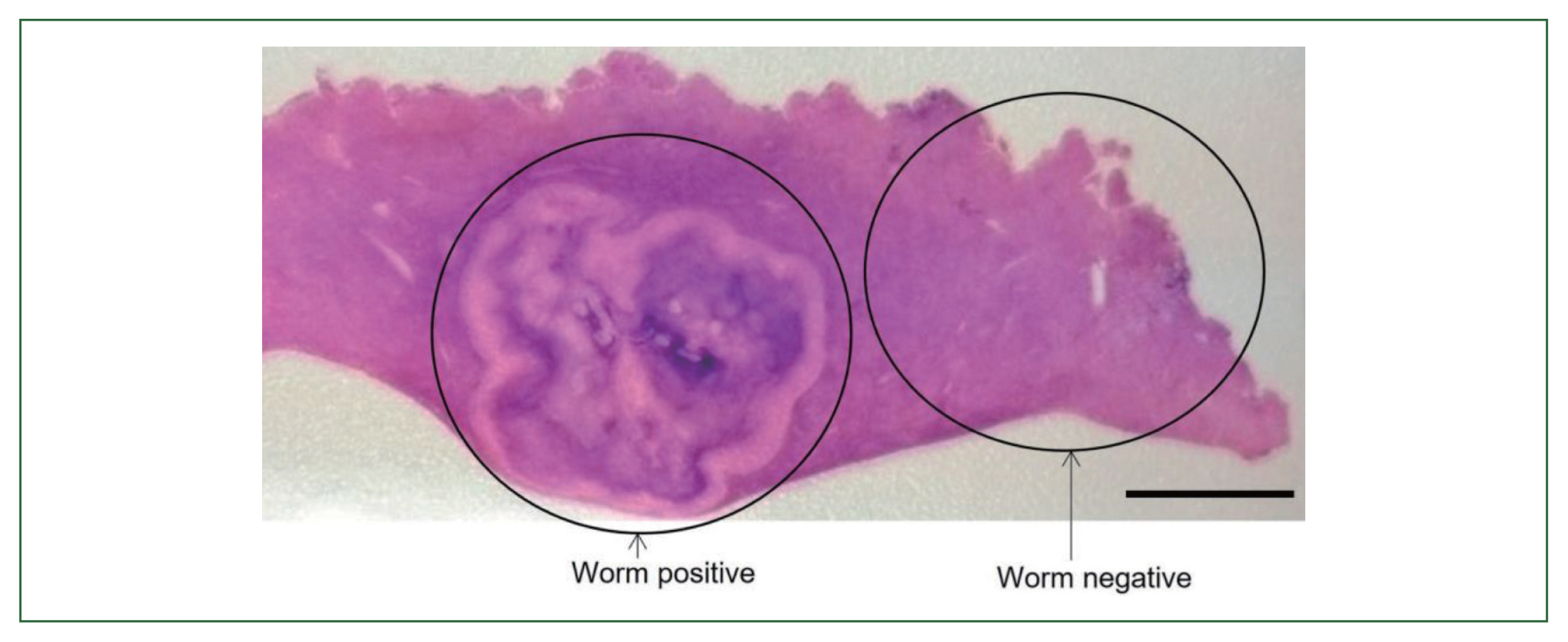
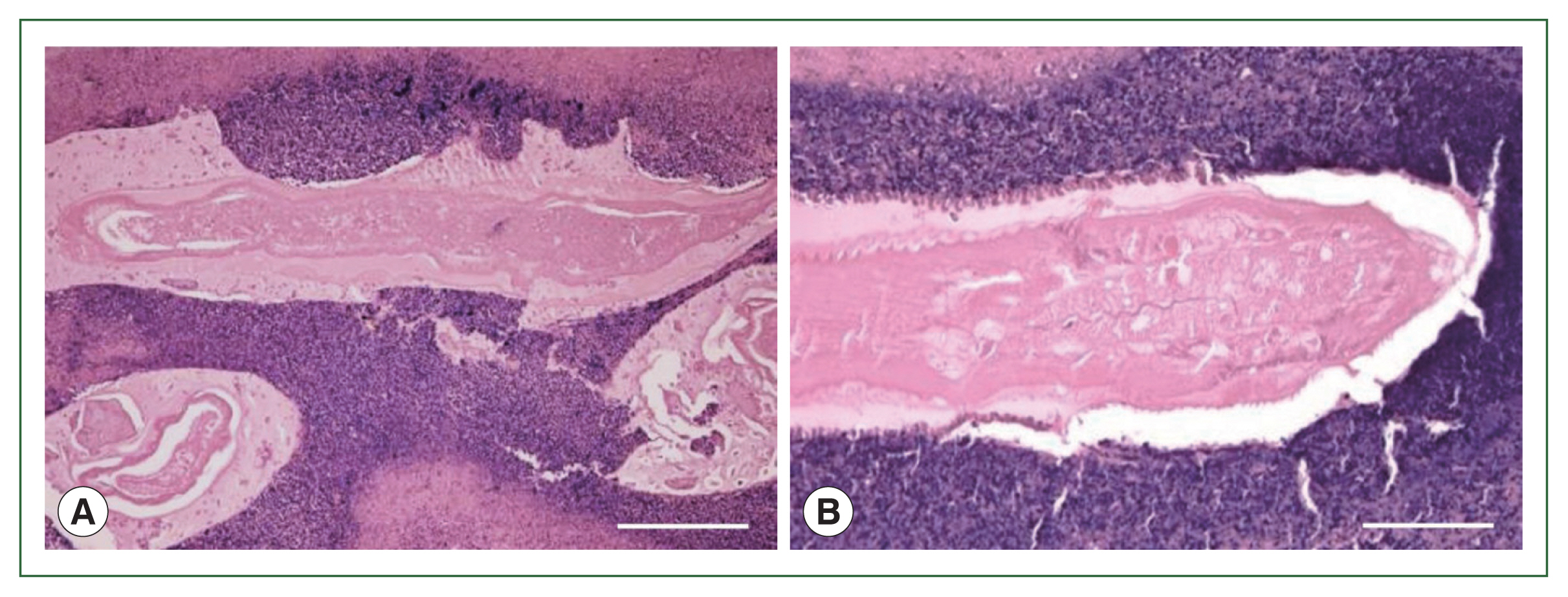
4). Necrotizing eosinophilic granulomatous connective tissues surrounding the larval nematode were visualized with hematoxylin and eosin staining, but were not clarified by Victorian-blue or elastica staining (
Figs. 5,
6). DNA samples were extracted from parasite-positive and -negative regions in paraffin-embedded sections using the DEXPAT reagent (TaKaRa, Shiga, Japan) and the QIAmp DNA Mini Kit (Qiagen GmbH, Hilden, Germany) to molecularly identify the larvae in the nematode-positive lesion. A nuclear DNA region of internal transcribed spacer (ITS)1, 5.8S rRNA and ITS2, and a mitochondrial DNA region of
COX2 are used to identify
Anisakis larvae. The
COX2 region was amplified by polymerase chain reaction (PCR) and sequenced in the present case. The primers used for
COX2 were 5′-TCAGGATTTTGGTTTGATGTTT-3′ and 5′-ATTCTCCATAAAACCTATACAC-3′ [
10]. A PCR reaction was performed under the following conditions: samples were denatured at 95°C for 3 min and then subjected to 40 cycles of 94°C for 30 sec, 48°C for 40 sec, and 72°C for 50 sec, with final extension at 72°C for 7 min on a thermocycler (GeneAmp PCR System 9700, Applied Bio-systems, Foster City, CA, USA). The PCR product (682 bp) was purified with a QIAquick PCR Purification Kit (Qiagen GmbH) and sequenced. Clustal X2 [
11] aligned sequences and BioEdit 7.0.5.3 manually edited computed sequences [
12]. All gaps were eliminated and
COX2 sequences were used for the phylogenetic analysis. A maximum likelihood (ML) analysis was performed with MEGA 6.0.6 [
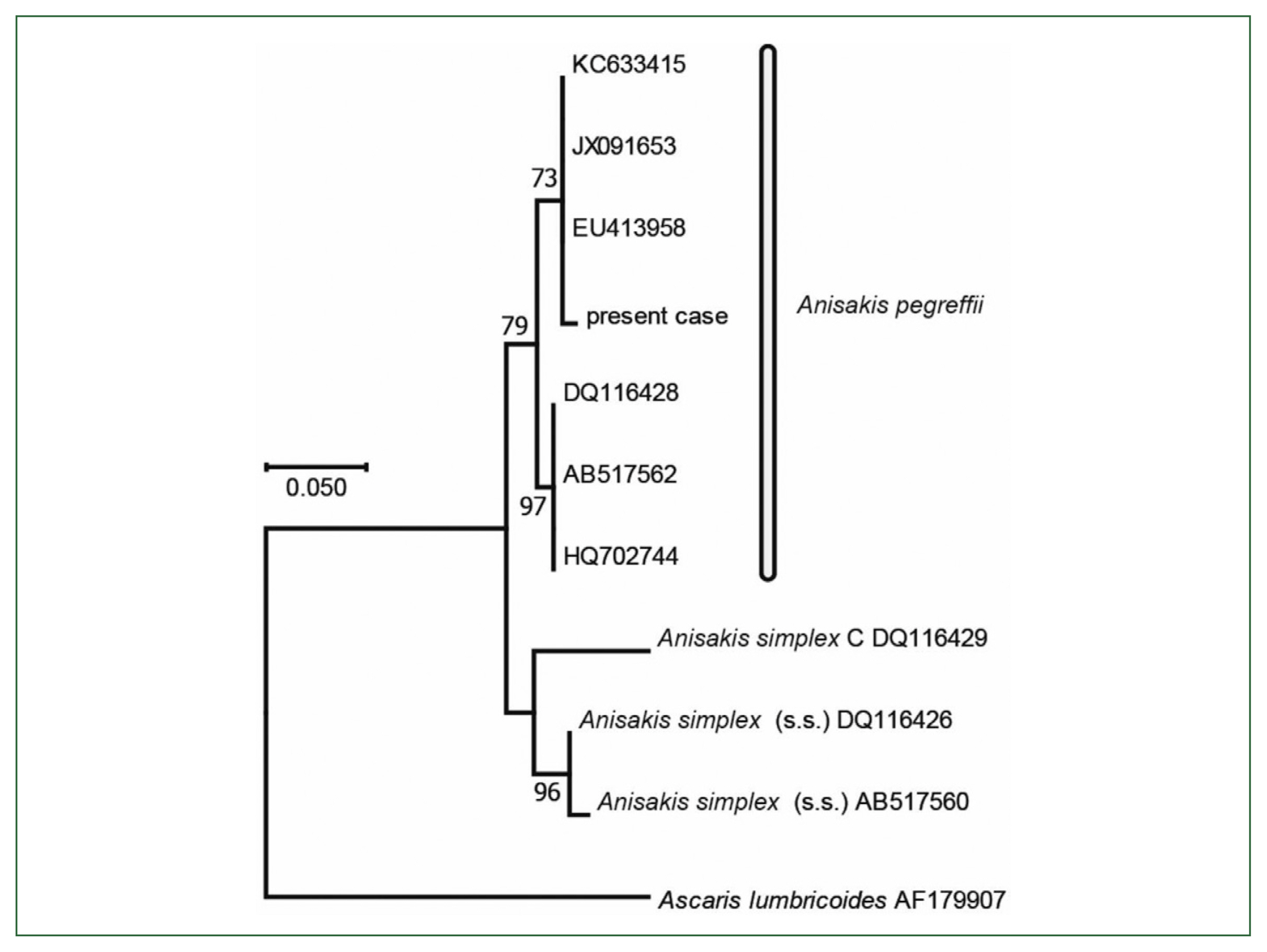
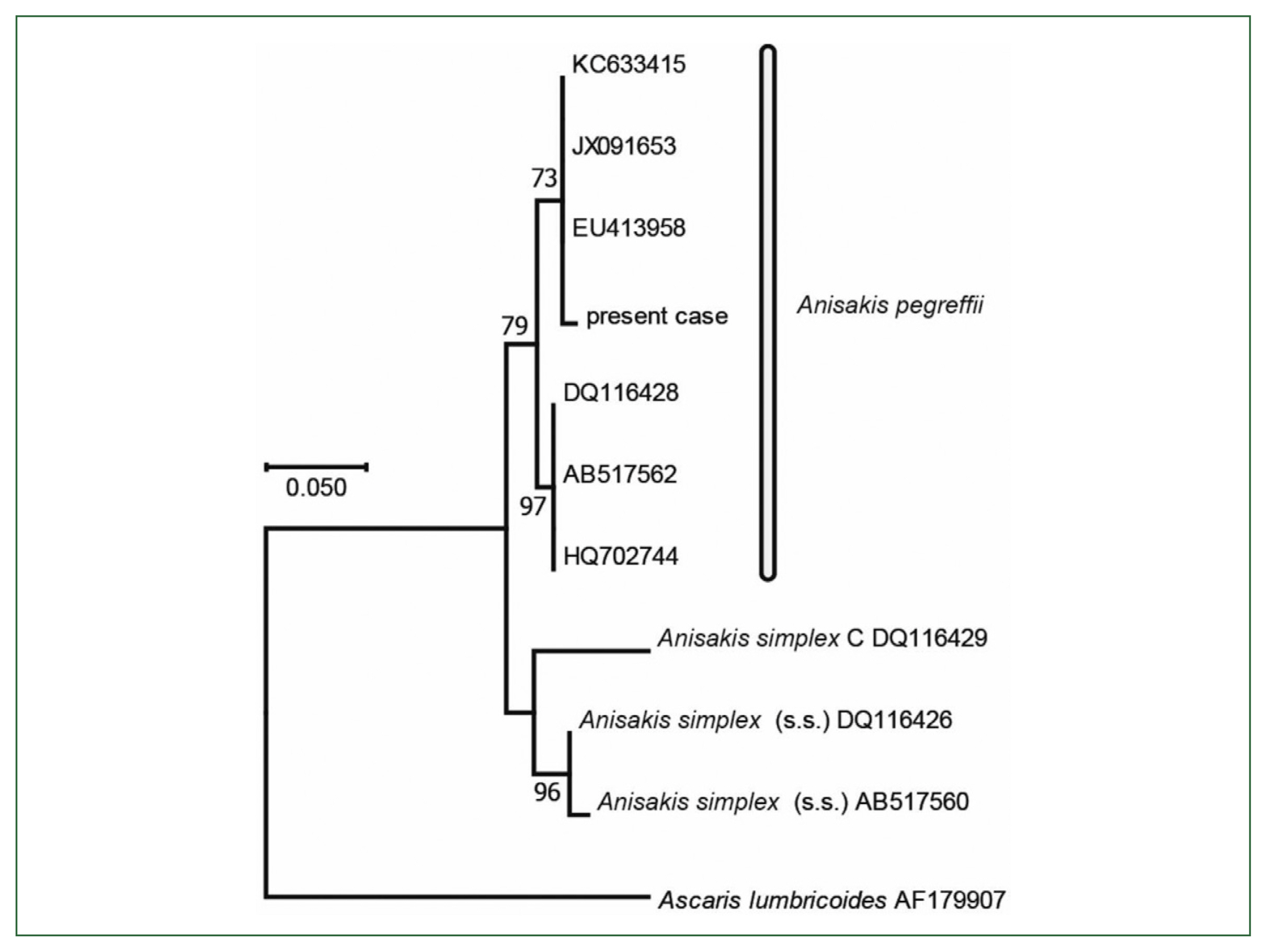
13]. The substitution model and optional parameter sets used were assessed with MEGA 6.0.6, and the most suitable sets were selected following the Akaike information criterion. The same datasets were used to construct 1,000 ML trees to calculate the bootstrap values. the final dataset contained 468 positions. Molecular and phylogenetic analysis of
COX2 (468 bp, partial sequence) sequences revealed
Anisakis larvae belonging to
A. pegreffii (
Fig. 6).
Discussion
Anisakidosis is one of the most common fishborne helminthic diseases in Japan, which is contracted by ingesting the nematode
Anisakis spp larvae, carried by marine fish [
1]. The dominant species in fish caught offshore Japan included
A. simplex (s.s.) and
A. pegreffii [
9]. A previous study revealed that significantly higher rates of
A. simplex (s.s.) penetrated agar than
A. pegreffii, indicating that its larvae can survive in acidic gastric juice to some extent and penetrate the stomach, small intestine, or colon of infected humans [
14]. Furthermore, in vitro and in vivo studies revealed that
A. pegreffii can pathogenically cause anisakidosis in humans when ingested, similar to
A. simplex (s.s.) [
15]. Recent cases in Italy indicated that
A. pegreffii is a major cause for concern in countries in which it is dominant [
16], similar to
A. simplex (s.s.) in Japan [
14]. The present case report is crucial because it describes a rare hepatic anisakidosis case caused by
A. pegreffii, which is mostly encountered in gastric, intestinal, and extra-gastrointestinal (ectopic) cases mainly caused by
A. simplex (s.s.) in Japan [
14]. Difficulties were associated with differentiating between liver cancer and parasitic granuloma solely based on imaging modalities. An accurate diagnosis is possible using PCR based on
COX2 genes subjected to a mtDNA sequence analysis although ectopic anisakidosis is challenging to diagnose using pathological specimens. A molecular/genetic methodological approach allows for the easy and rapid
Anisakis spp. identification in formalin-fixed and paraffin-embedded tissues obtained from gastric or intestinal human anisakidosis cases [
16,
17]. Laparoscopic partial hepatectomy for a pathological examination and the genetic anisakidosis identification by PCR were useful for achieving a final diagnosis even when degenerative granuloma surrounding larval bodies necrotizing in the resected specimen collapses with time. Only 8 hepatic anisakidosis cases have been reported from Japanese people who frequently consume undercooked fish [
18,
19]. In conclusion, the present results emphasize the rarity of ectopic anisakidosis, which may help identify and characterize unknown
Anisakis species in biopsied histological sections misdiagnosed as liver cancer.
Notes
-
Author contributions
Conceptualization: Yamada M, Murakoshi F, Ikoma H
Investigation: Inamori O
Methodology: Murakoshi F
Supervision: Yanagisawa A, Konishi E
Visualization: Ikoma H
Writing – original draft: Yamada M, Murakoshi F
Writing – review & editing: Yamada M, Murakoshi F
-
We have no conflict of interest related to this work.
Acknowledgments
This study was performed in accordance with the ethical standards of the Institutional Review Board of the Kyoto Prefectural University of Medicine and with the Declaration of Helsinki as revised in 2013 and an informed consent for publication was obtained from the patient.
Fig. 1Abdominal CT. (A) Early stage of arterial phase. Contrast CT after 30 sec. (B) Equilibrium stage of arterial phase. Contrast CT after 180 sec. Arrows show a low-density area of 20 mm in size in segment 6 of the liver. CT, computed tomography.
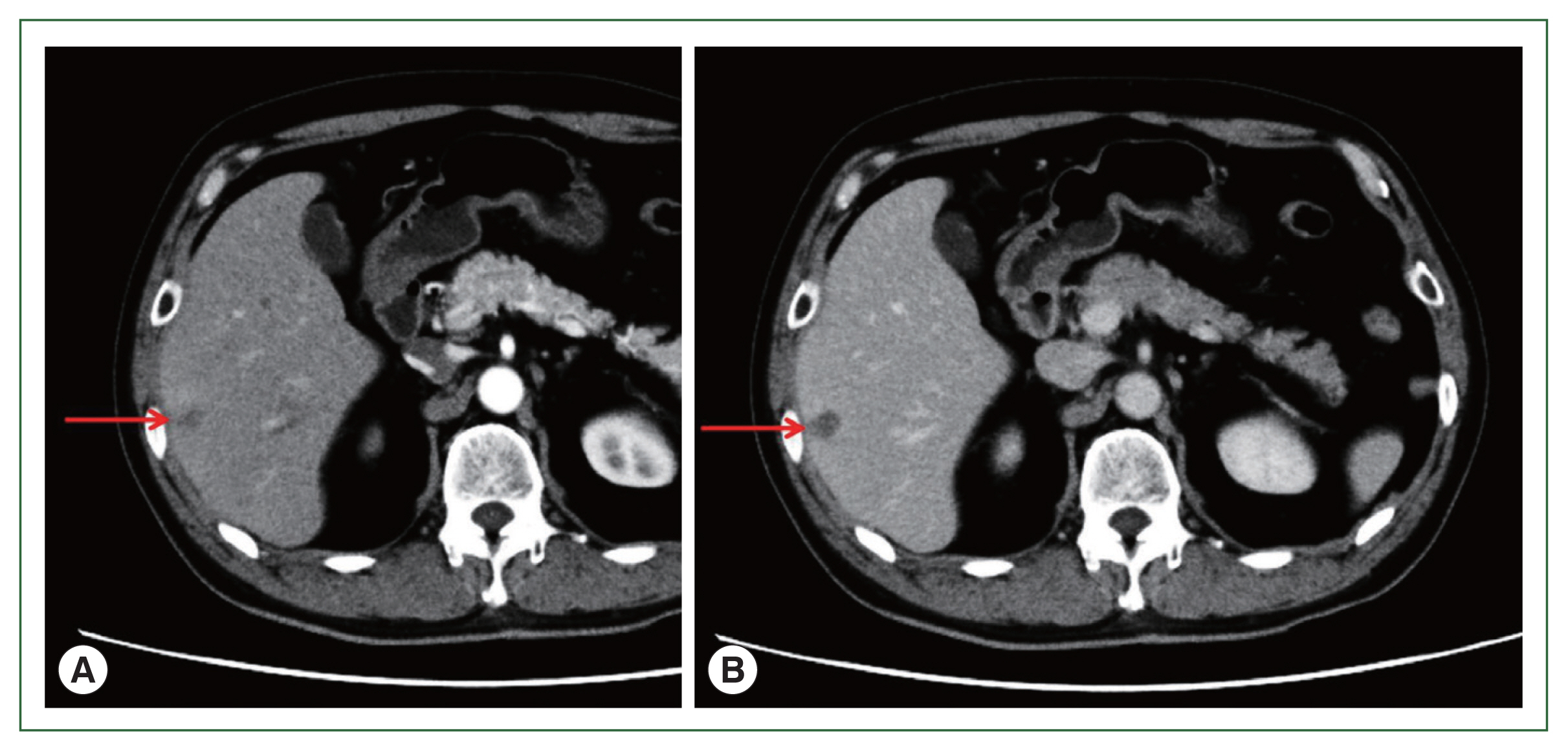
Fig. 2(A) EOB-MRI. Left: Early stage of arterial phase. Right: Equilibrium stage of arterial phase. Arrows show a low-density area of 20 mm in size in segment 6 of the liver. (B) EOB-MRI. Liver cell phase of arterial phase. Arrow shows low-density signal regions that showed lighter color than those of the early and equilibrium stages. EOB-MRI, ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging.
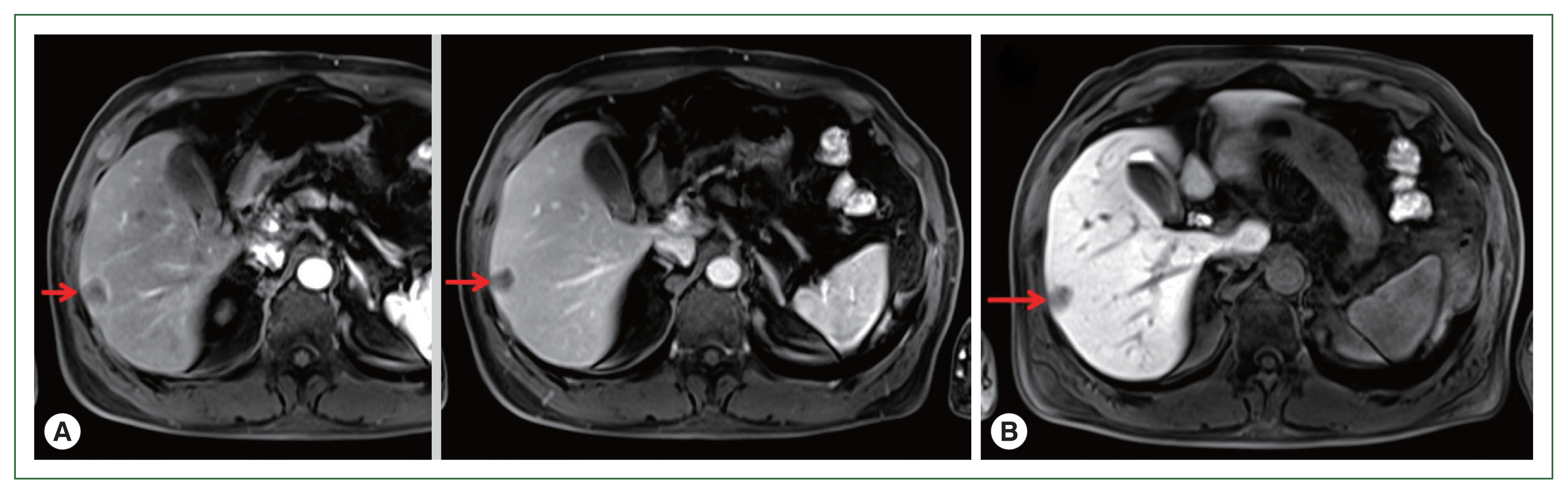
Fig. 3Necrotizing granuloma of the S6 lesions in the liver section partially resected.
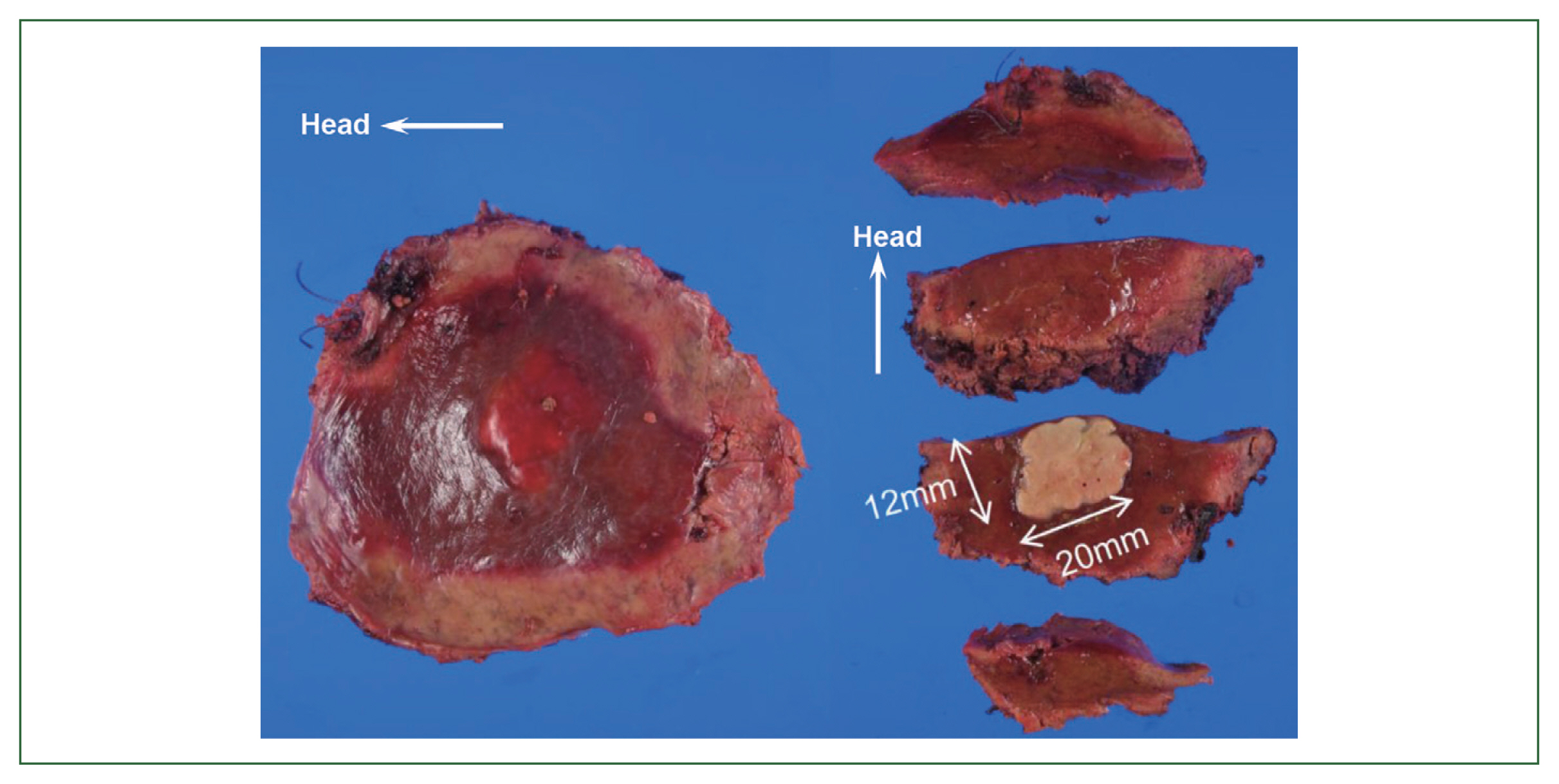
Fig. 4Low magnification of the tissue section infected with the worm in the S6 lesion of the liver.Bar=1 cm.
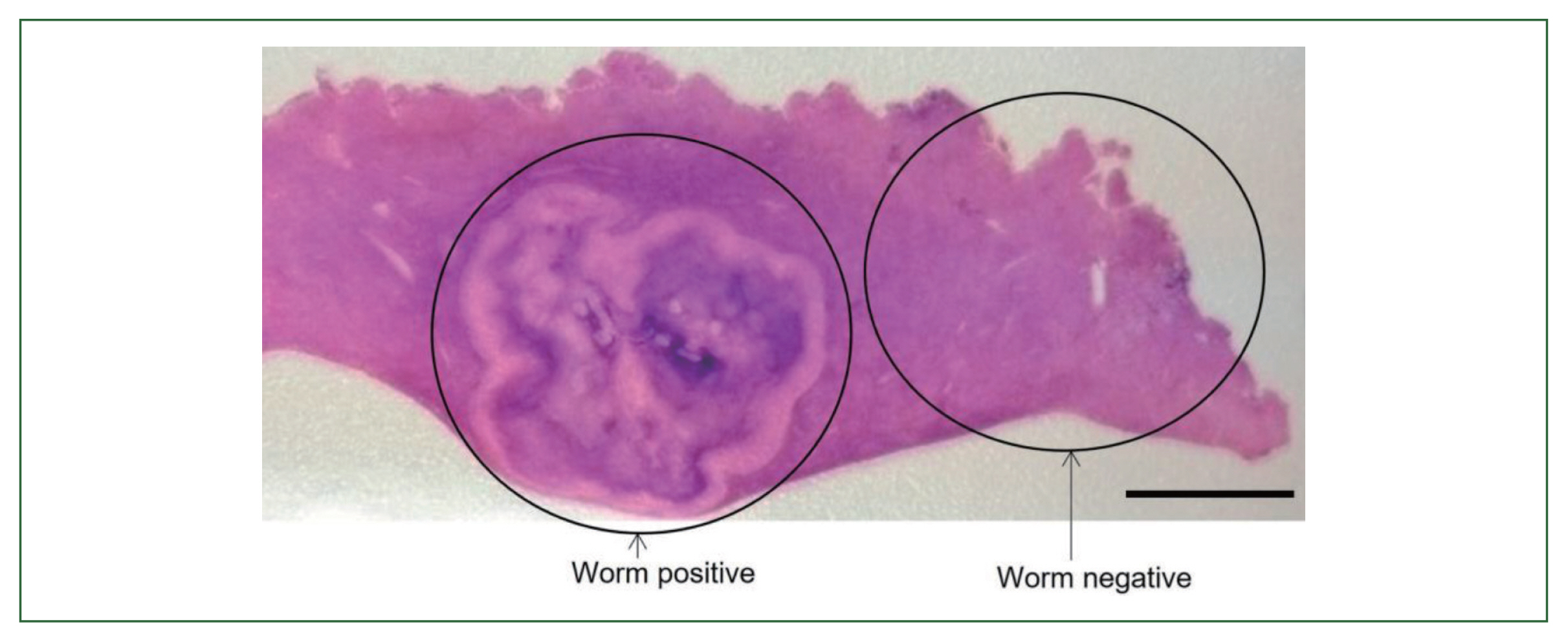
Fig. 5(A) Larval nematode, Anisakis sp. in the tissue section of the S6 lesion of the liver. Magification (×100). Bar=200 μm. (B) Higher magnification of the larval nematode, Anisakis sp. in the tissue section of the S6 lesion of the liver. Magification (×200). Bar=100 μm.
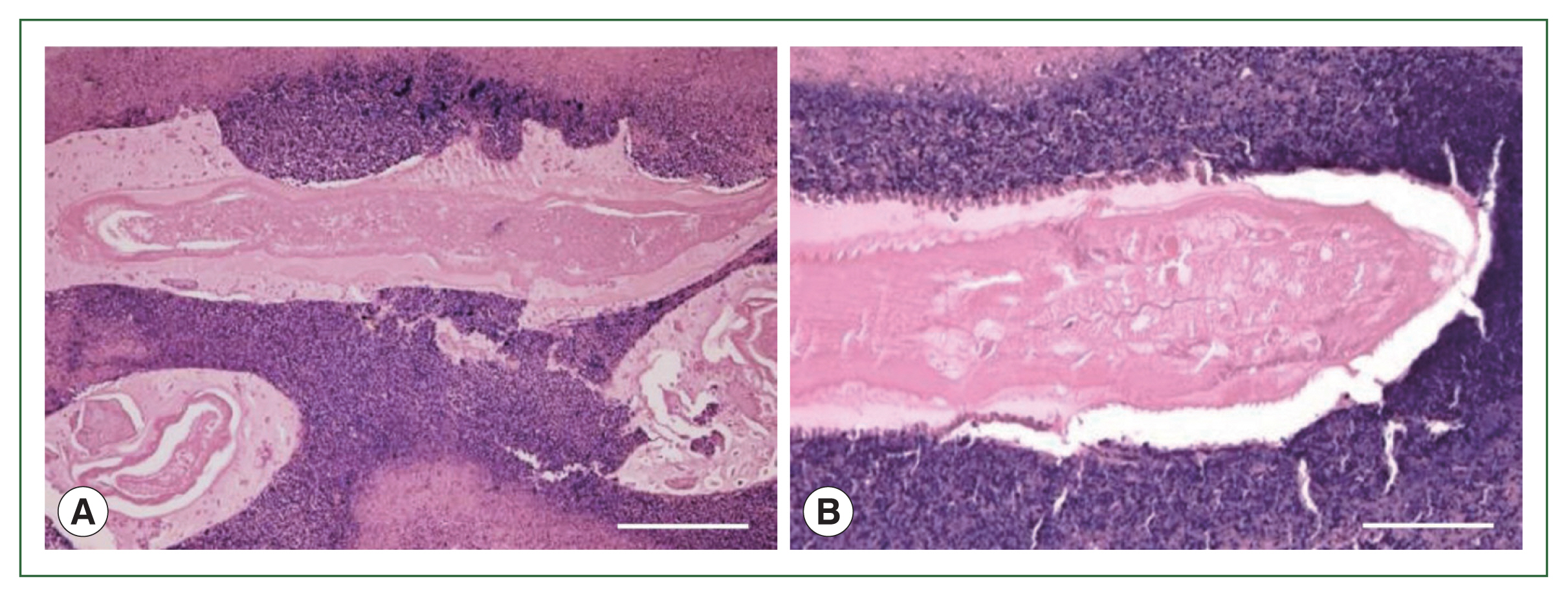
Fig. 6Phylogenetic tree based on partial sequences of the COX2 sequence constructed using the maximum likelihood method with 468 nucleotides without gaps. Substitution model and optional parameters=GTR+Γ. Only bootstrap values of >50% from 1,000 pseudo-replicates are shown.
References
- 1. Chai JY, Murrell KD, Lambery AJ. Fish-borne parasitic zoonoses: status and issues. Int J Parasitol 2005;35(11–12):1233-1254.
https://doi.org/10.1016/j.ijpara.2005.07.013
- 2. Sohn WM, Chai JY. Anisakiosis (anisakidosis). In Palmer SR, Soulsby L, Torgerson PR, Brown WG eds, Oxford Textbook of Zoonoses. 2nd ed. Oxford University Press; Oxford, UK. 2011, pp 774-786.
- 3. Ishikura H. 5. Anisakiasis. (2) Clinical pathology and epidemiology. In Otsuru M, Kamegai S, Hayashi S eds, Progress of Medical Parasitology in Japan. 8:Meguro Parasitological Museum; Tokyo, Japan. 2003, pp 451-473.
- 4. Takahashi S, Ishikura H, Kikuchi K. Anisakidosis: global point of view. In Ishikura H ed, Host Response to International Parasitic Zoonoses. Springer-Verlag; New York, USA. 1998, pp 109-120.
- 5. Takabe K, Ohki S, Kunihiro O, Sakashita T, Endo I, et al. Anisakidosis: a cause of intestinal obstruction from eating sushi. Am J Gastroenterol 1998;93(7):1172-1173.
https://doi.org/10.1111/j.1572-0241.1998.00356.x
- 6. Arizono N, Miura T, Yamada M, Tegoshi T, Onishi K. Human infection with Pseudoterranova azarasi roundworm. Emeg Infect Dis 2011;17(3):555-556.
https://doi.org/10.3201/eid1703.101350
- 7. Mitsuboshi A, Yamaguchi H, Ito Y, Mizuno T, Tokoro M, et al. Extra-gastrointestinal anisakidosis caused by Pseudoterranova azarasi manifesting as strangulated inguinal hernia. Parasitol Int 2017;66(6):810-812.
https://doi.org/10.1016/j.parint.2017.09.008
- 8. Mattiucci S, Nascetti G. Advances and trends in the molecular systematics of anisakid nematodes, with implications for their evolutionary ecology and host-parasite co-evolutionary processes. Adv Parasitol 2008;66:47-148.
https://doi.org/10.1016/S0065-308X(08)00202-9
- 9. Umehara A, Kawakami Y, Araki J, Uchida A. Molecular identification of the etiological agent of the human anisakiasis in Japan. Parasitol Int 2007;56(3):211-215.
https://doi.org/10.1016/j.parint.2007.02.005
- 10. Valentini A, Matticci S, Bondanelli P, Webb SC, Mignucci-Giannone AA, et al. Genetic relationships among Anisakis species (Nematoda: Anisakidae) inferred from mitochondrial cox2 sequences and comparison with allozyme data. J Parasitol 2006;92(1):156-166.
https://doi.org/10.1645/GE-3504.1
- 11. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettingen PA, et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007;23(21):2947-2948.
https://doi.org/10.1093/bioinformatics/btm404
- 12. Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series 1999;41:95-98.
- 13. Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis Version 6.0. Mol Biol Evol 2013;30(12):2725-2729.
https://doi.org/10.1093/molbev/mst197
- 14. Arizono N, Yamada M, Tegoshi T, Yoshikawa M.
Anisakis simplex sense stricto and Anisakis pegreffii: biological characteristics and pathogenic potential in human anisakiasis. Foodborne Pathog Dis 2012;9(6):517-521.
https://doi.org/10.1089/fpd.2011.1076
- 15. Jeon CH, Kim JH. Pathogenic potential of the two sibling species, Anisakis simplex (s.s.) and Anisakis pegreffii (Nematoda: Anisakidae): in vitro and in vivo studies. Biomed Res Int 2015;2015:983656.
https://doi.org/10.1155/2015/983656
- 16. Mattiucci S, Paoletti M, Borrini F, Palumbo M, Palmieri RM, et al. First molecular identification of the zoonotic parasite Anisakis pegreffi (Nematoda: Anisakidae) in a paraffin-embedded granuloma taken from a case of human intestinal anisakiasis in Italy. BMC Infect Dis 2011;11:82.
https://doi.org/10.1186/1471-2334-11-82
- 17. Mladineo I, Popović M, Drmić-Hofman I, Poljak V. A case report of Anisakis pegreffii (Nematoda, Anisakidae) identified from archival paraffin sections of a Croatian patient. BMC Infect Dis 2016;16:42.
https://doi.org/10.1186/s12879-016-1401-x
- 18. Kagei N, Orikasa H, Hori E, Sannomiya A, Yasumura Y. A case of hepatic anisakiasis with a literal survey for extra-gastrointestinal anisakiasis. Jpn J Parasitol 1995;44:346-351.
- 19. Murata Y, Ando K, Usui M, Sugiyama H, Hayashi A, et al. A case of hepatic anisakiasis caused by Pseudoterranova decipiens mimicking metastatic liver cancer. BMC Infect Dis 2018;18(1):619.
https://doi.org/10.1186/s12879-018-3540-8
Citations
Citations to this article as recorded by

- Anisakidae and Anisakidosis: A Public Health Perspective
Diana Nonković, Vanja Tešić, Vida Šimat, Svjetlana Karabuva, Alan Medić, Jerko Hrabar
Pathogens.2025; 14(3): 217. CrossRef - Screening of Anisakis-Related Allergies and Associated Factors in a Mediterranean Community Characterized by High Seafood Consumption
Santo Fruscione, Maria Barrale, Maurizio Zarcone, Davide Alba, Barbara Ravazzolo, Miriam Belluzzo, Rosa Onida, Gaetano Cammilleri, Antonella Costa, Vincenzo Ferrantelli, Alessandra Savatteri, Daniele Domenico De Bella, Salvatore Pipitone, Alida D’Atria, A
Foods.2024; 13(17): 2821. CrossRef
 , Fumi Murakoshi1,2
, Fumi Murakoshi1,2 , Hisashi Ikoma3
, Hisashi Ikoma3 , Osamu Inamori4
, Osamu Inamori4 , Akio Yanagisawa4
, Akio Yanagisawa4 , Eiichi Konishi4
, Eiichi Konishi4